A unified cell biological perspective on axon-myelin injury
- PMID: 25092654
- PMCID: PMC4121977
- DOI: 10.1083/jcb.201404154
A unified cell biological perspective on axon-myelin injury
Abstract
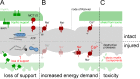
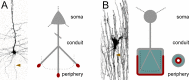
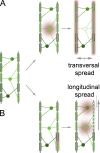
Demyelination and axon loss are pathological hallmarks of the neuroinflammatory disorder multiple sclerosis (MS). Although we have an increasingly detailed understanding of how immune cells can damage axons and myelin individually, we lack a unified view of how the axon-myelin unit as a whole is affected by immune-mediated attack. In this review, we propose that as a result of the tight cell biological interconnection of axons and myelin, damage to either can spread, which might convert a local inflammatory disease process early in MS into the global progressive disorder seen during later stages. This mode of spreading could also apply to other neurological disorders.
© 2014 Simons et al.
Figures


References
-
- Aboul-Enein, F., Rauschka H., Kornek B., Stadelmann C., Stefferl A., Brück W., Lucchinetti C., Schmidbauer M., Jellinger K., and Lassmann H.. 2003. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J. Neuropathol. Exp. Neurol. 62:25–33 - PubMed
-
- Aggarwal, S., Snaidero N., Pähler G., Frey S., Sánchez P., Zweckstetter M., Janshoff A., Schneider A., Weil M.T., Schaap I.A., et al. . 2013. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 11:e1001577 10.1371/journal.pbio.1001577 - DOI - PMC - PubMed
-
- Arthur-Farraj, P., Mirsky R., Parkinson D.B., and Jessen K.R.. 2006. A double point mutation in the DNA-binding region of Egr2 switches its function from inhibition to induction of proliferation: A potential contribution to the development of congenital hypomyelinating neuropathy. Neurobiol. Dis. 24:159–169 10.1016/j.nbd.2006.06.006 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

