Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval
- PMID: 25092702
- PMCID: PMC4187937
- DOI: 10.1128/AAC.02667-14
Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval
Abstract
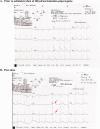
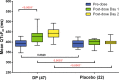
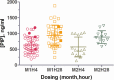
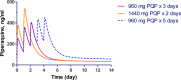
Dihydroartemisinin-piperaquine, the current first-line drug for uncomplicated malaria caused by Plasmodium falciparum and Plasmodium vivax in Cambodia, was previously shown to be of benefit as malaria chemoprophylaxis when administered as a monthly 3-day regimen. We sought to evaluate the protective efficacy of a compressed monthly 2-day treatment course in the Royal Cambodian Armed Forces. The safety and efficacy of a monthly 2-day dosing regimen of dihydroartemisinin-piperaquine were evaluated in a two-arm, randomized, double-blind, placebo-controlled cohort study with 2:1 treatment allocation. Healthy military volunteers in areas along the Thai-Cambodian border where there is a high risk of malaria were administered two consecutive daily doses of 180 mg dihydroartemisinin and 1,440 mg piperaquine within 30 min to 3 h of a meal once per month for a planned 4-month period with periodic electrocardiographic and pharmacokinetic assessment. The study was halted after only 6 weeks (69 of 231 projected volunteers enrolled) when four volunteers met a prespecified cardiac safety endpoint of QTcF (Fridericia's formula for correct QT interval) prolongation of >500 ms. The pharmacodynamic effect on the surface electrocardiogram (ECG) peaked approximately 4 h after piperaquine dosing and lasted 4 to 8 h. Unblinded review by the data safety monitoring board revealed mean QTcF prolongation of 46 ms over placebo at the maximum concentration of drug in serum (Cmax) on day 2. Given that dihydroartemisinin-piperaquine is one of the few remaining effective antimalarial agents in Cambodia, compressed 2-day treatment courses of dihydroartemisinin-piperaquine are best avoided until the clinical significance of these findings are more thoroughly evaluated. Because ECG monitoring is often unavailable in areas where malaria is endemic, repolarization risk could be mitigated by using conventional 3-day regimens, fasting, and avoidance of repeated dosing or coadministration with other QT-prolonging medications. (This study has been registered at ClinicalTrials.gov under registration no. NCT01624337.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- World Health Organization. 2013. World malaria report 2013. World Health Organization, Geneva, Switzerland
-
- Bethell D, Se Y, Lon C, Tyner S, Saunders D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P, Schaecher K, Ruttvisutinunt W, Lin J, Kuntawungin W, Gosi P, Timmermans A, Smith B, Socheat D, Fukuda MM. 2011. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One 6:e19283. 10.1371/journal.pone.0019283 - DOI - PMC - PubMed
-
- Zwang J, Ashley EA, Karema C, D'Alessandro U, Smithuis F, Dorsey G, Janssens B, Mayxay M, Newton P, Singhasivanon P, Stepniewska K, White NJ, Nosten F. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. 10.1371/journal.pone.0006358 - DOI - PMC - PubMed
-
- Naing C, Mak JW, Aung K, Wong JY. 2013. Efficacy and safety of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in endemic countries: meta-analysis of randomised controlled studies. Trans. R. Soc. Trop. Med. Hyg. 107:65–73. 10.1093/trstmh/trs019 - DOI - PubMed
-
- Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. 2013. Efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob. Agents Chemother. 57:818–826. 10.1128/AAC.00686-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

