Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter
- PMID: 25092770
- PMCID: PMC4197864
- DOI: 10.15252/emmm.201404077
Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter
Abstract
In this report, we describe the development of a modified adeno-associated virus (AAV) capsid and promoter for transduction of retinal ON-bipolar cells. The bipolar cells, which are post-synaptic to the photoreceptors, are important retinal targets for both basic and preclinical research. In particular, a therapeutic strategy under investigation for advanced forms of blindness involves using optogenetic molecules to render ON-bipolar cells light-sensitive. Currently, delivery of adequate levels of gene expression is a limiting step for this approach. The synthetic AAV capsid and promoter described here achieves high level of optogenetic transgene expression in ON-bipolar cells. This evokes high-frequency (~100 Hz) spiking responses in ganglion cells of previously blind, rd1, mice. Our vector is a promising vehicle for further development toward potential clinical use.
Keywords: adeno‐associated virus; capsid library; multi‐electrode array; optogenetics; promoter optimization.
© 2014 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
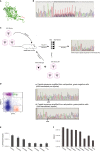
A The secondary structure of the AAV capsid 8 (PDB:2QA0) with the region targeted for mutation highlighted in red.
B Electropherogram of AAV8 capsid sequence from nucleotide 1731 to 1800 containing the mutated region between amino acids 585 and 593.
C The schema for AAV capsid variant isolation: In round 1, the AAV library, “AAV2/8lib-dsRed” was subretinally injected into GRM6-GFP mice. After 4 weeks, the retinas were dissociated and green cells isolated by FACS. In round 2, the viral library filtered through round 1 was used to create a titrated AAV2/8lib-dsRed library R1. This was mixed with a non-mutated AAV2/8-dsRed, serving as competing virus, and the viral mix cosubretinally injected into GRM6-GFP mice. From round 2, green/red and red-only cells were isolated by FACS for further analysis of sequence variants.
D The electropherograms of AAV capsid sequences amplified by PCR from the cells with only non-mutated sequences isolated from red-only cells (a) and non-mutated as well as variant sequences present in the red/green population (b).
E, F The titers of the novel viruses were determined by luciferase assay for small-scale preparations (E) and RT-qPCR for large-scale preparations (F).

A, B Representative 20× confocal images of immunostained vibratome sections from retinas of mice subretinally injected (A) or intravitreally injected (B) with viruses. The upper panels show AAV2/8(EF1α-EGFP) injected retinas, and the lower panels show AAV2/8BP2(EF1α-EGFP) injected retinas. The retinas are stained for EGFP (green), cell nuclei (gray), and for the inner plexiform layer strata using choline acetyltransferase (ChAT) (magenta). POS, photoreceptor outer segments; ONL, outer nuclear layer; OPL, outer plexiform layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
C The number of genome copies per cell was estimated for purified AAV2/8BP2 compared to AAV2/8 following transduction of HEK293 cells.
D Cell counts from FACS analysis of retinas from mice (n = 4) subretinally injected with AAV2/8(EF1α-EGFP) or AAV2/8BP2(EF1α-EGFP).
E RT-qPCR on RNA from the sorted cells used to determine bipolar cell gene expression levels with 120% increase in Grm6 expression (P = 0.05) and 67% increase in TrpM1L expression (P = 0.04) in the AAV2/8BP2(EF1α-EGFP) cell pool.
F Equivalent expression levels measured between cell pools for the cone photoreceptor genes Opnmwl and Opnswl.
G Representative 40× confocal images of sections from the retinas of mice subretinally injected with AAV2/8(4 × GRM6-EGFP) (upper panel) and AAV2/8BP2(4 × GRM6-EGFP) (lower panel). The panel on the left shows sections stained for EGFP (green) and cell nuclei (blue), while the panel on the right shows live fluorescence images.
H Retinas from mice that were intravitreally injected were similarly analyzed.
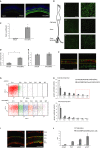
A 10× images of sections from WT mice subretinally injected with AAV2/8(4 × GRM6-EGFP) and AAV2/8BP2(4 × GRM6-EGFP). The sections were stained for EGFP protein expression (green) and for the cell nuclei (blue).
B Unfixed and unstained whole-mount images of the cell body, axonal and dendritic regions of the bipolar cells from the injected retinas taken at 40× magnification.
C The fluorescent spot counts on local z-stack projections spanning the cell body of these retinas (n = 6, P = 0.01).
D–F Cell counts of colocalization of the red (TrpM1L) and green (EGFP) channels (n = 4), with the counts normalized to total red cells (D, P = 0.04) or to total green cells (E). A representative image of the labeled retinal sections used for cell counting is shown (F).
G FACS analysis of subretinally and intravitreally injected retinas carried out at 3 weeks post-injection. Representative dot-plot of the fluorescence intensity range for the subretinally injected retinas with GFP versus APC-a (n = 6). AAV2/8 injected retina is shown in the top panel while AAV2/8BP2-injected retina is shown in the bottom panel.
H The FACS intensity range divided across seven regions, Fr8 to Fr14, with the percentage of fluorescent cells shown on the y-axis.
I A representative 40× image of a section from subretinally injected retinas stained for EGFP (green), cell nuclei (blue), and the rod-bipolar cell marker PKCα (red).
J Expression of kcng4, lhx4, prkcα, and grm6 in cells of sorted fractions Fr12 to Fr14 (highlighted by a red box in the upper panel of Fig 3H) relative to expression in unsorted cells from total retina.
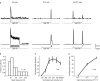
Light responses to full field stimulation from six example cells. Both ON, OFF and ON-OFF cells are recorded.
Histogram of peak firing frequencies.
Response to increasing spots.
Firing rate as a function of light intensity.
References
-
- Agbandje-McKenna M, Kleinschmidt J. AAV capsid structure and cell interactions. Methods Mol Biol. 2011;807:47–92. - PubMed
-
- Bainbridge JWB, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

