Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance)
- PMID: 25092775
- PMCID: PMC4268249
- DOI: 10.1200/JCO.2014.57.0572
Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance)
Abstract
Purpose: One third of patients with triple-negative breast cancer (TNBC) achieve pathologic complete response (pCR) with standard neoadjuvant chemotherapy (NACT). CALGB 40603 (Alliance), a 2 × 2 factorial, open-label, randomized phase II trial, evaluated the impact of adding carboplatin and/or bevacizumab.
Patients and methods: Patients (N = 443) with stage II to III TNBC received paclitaxel 80 mg/m(2) once per week (wP) for 12 weeks, followed by doxorubicin plus cyclophosphamide once every 2 weeks (ddAC) for four cycles, and were randomly assigned to concurrent carboplatin (area under curve 6) once every 3 weeks for four cycles and/or bevacizumab 10 mg/kg once every 2 weeks for nine cycles. Effects of adding these agents on pCR breast (ypT0/is), pCR breast/axilla (ypT0/isN0), treatment delivery, and toxicities were analyzed.
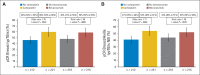
Results: Patients assigned to either carboplatin or bevacizumab were less likely to complete wP and ddAC without skipped doses, dose modification, or early discontinuation resulting from toxicity. Grade ≥ 3 neutropenia and thrombocytopenia were more common with carboplatin, as were hypertension, infection, thromboembolic events, bleeding, and postoperative complications with bevacizumab. Employing one-sided P values, addition of either carboplatin (60% v 44%; P = .0018) or bevacizumab (59% v 48%; P = .0089) significantly increased pCR breast, whereas only carboplatin (54% v 41%; P = .0029) significantly raised pCR breast/axilla. More-than-additive interactions between the two agents could not be demonstrated.
Conclusion: In stage II to III TNBC, addition of either carboplatin or bevacizumab to NACT increased pCR rates, but whether this will improve relapse-free or overall survival is unknown. Given results from recently reported adjuvant trials, further investigation of bevacizumab in this setting is unlikely, but the role of carboplatin could be evaluated in definitive studies, ideally limited to biologically defined patient subsets most likely to benefit from this agent.
Trial registration: ClinicalTrials.gov NCT00861705.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures


Comment in
-
Defining a role and predicting benefit from platinum-based therapy in breast cancer: an evolving story.J Clin Oncol. 2015 Jan 1;33(1):1-3. doi: 10.1200/JCO.2014.57.7890. Epub 2014 Oct 20. J Clin Oncol. 2015. PMID: 25332246 No abstract available.
-
[Neoadjuvant chemotherapy in triple-negative breast cancer: is there a role for platinum?].Bull Cancer. 2014 Dec;101(12):1063. doi: 10.1684/bdc.2014.2051. Bull Cancer. 2014. PMID: 25776517 French. No abstract available.
-
How successful is NST in increasing breast-conserving surgery rates in TNBC patients?Bull Am Coll Surg. 2016 Apr;101(4):40-2. Bull Am Coll Surg. 2016. PMID: 27337767 No abstract available.
References
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. - PubMed
-
- Decatris MP, Sundar S, O'Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: Current status. Cancer Treat Rev. 2004;30:53–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- CA076001/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA025224/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- CA32102/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

