Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains
- PMID: 25093327
- PMCID: PMC4122339
- DOI: 10.1371/journal.pbio.1001921
Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains
Abstract
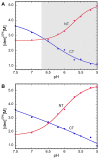
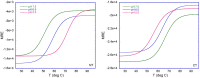
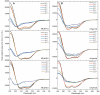
Spider silk fibers are produced from soluble proteins (spidroins) under ambient conditions in a complex but poorly understood process. Spidroins are highly repetitive in sequence but capped by nonrepetitive N- and C-terminal domains (NT and CT) that are suggested to regulate fiber conversion in similar manners. By using ion selective microelectrodes we found that the pH gradient in the silk gland is much broader than previously known. Surprisingly, the terminal domains respond in opposite ways when pH is decreased from 7 to 5: Urea denaturation and temperature stability assays show that NT dimers get significantly stabilized and then lock the spidroins into multimers, whereas CT on the other hand is destabilized and unfolds into ThT-positive β-sheet amyloid fibrils, which can trigger fiber formation. There is a high carbon dioxide pressure (pCO2) in distal parts of the gland, and a CO2 analogue interacts with buried regions in CT as determined by nuclear magnetic resonance (NMR) spectroscopy. Activity staining of histological sections and inhibition experiments reveal that the pH gradient is created by carbonic anhydrase. Carbonic anhydrase activity emerges in the same region of the gland as the opposite effects on NT and CT stability occur. These synchronous events suggest a novel CO2 and proton-dependent lock and trigger mechanism of spider silk formation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Simmons AH, Michal CA, Jelinski LW (1996) Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 271(5245): 84–87. - PubMed
-
- Hagn F, Eisoldt L, Hardy J, Vendrely C, Coles M, et al. (2010) A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 465: 239–242. - PubMed
-
- Askarieh G, Hedhammar M, Nordling K, Saenz A, Casals C, et al. (2010) Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 465: 236–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

