Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease
- PMID: 25093460
- PMCID: PMC4122355
- DOI: 10.1371/journal.pbio.1001923
Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease
Abstract
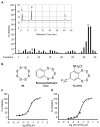
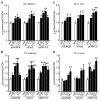
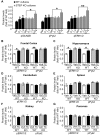
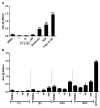
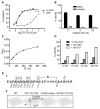
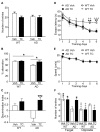
STEP (STriatal-Enriched protein tyrosine Phosphatase) is a neuron-specific phosphatase that regulates N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) trafficking, as well as ERK1/2, p38, Fyn, and Pyk2 activity. STEP is overactive in several neuropsychiatric and neurodegenerative disorders, including Alzheimer's disease (AD). The increase in STEP activity likely disrupts synaptic function and contributes to the cognitive deficits in AD. AD mice lacking STEP have restored levels of glutamate receptors on synaptosomal membranes and improved cognitive function, results that suggest STEP as a novel therapeutic target for AD. Here we describe the first large-scale effort to identify and characterize small-molecule STEP inhibitors. We identified the benzopentathiepin 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin-6-amine hydrochloride (known as TC-2153) as an inhibitor of STEP with an IC50 of 24.6 nM. TC-2153 represents a novel class of PTP inhibitors based upon a cyclic polysulfide pharmacophore that forms a reversible covalent bond with the catalytic cysteine in STEP. In cell-based secondary assays, TC-2153 increased tyrosine phosphorylation of STEP substrates ERK1/2, Pyk2, and GluN2B, and exhibited no toxicity in cortical cultures. Validation and specificity experiments performed in wild-type (WT) and STEP knockout (KO) cortical cells and in vivo in WT and STEP KO mice suggest specificity of inhibitors towards STEP compared to highly homologous tyrosine phosphatases. Furthermore, TC-2153 improved cognitive function in several cognitive tasks in 6- and 12-mo-old triple transgenic AD (3xTg-AD) mice, with no change in beta amyloid and phospho-tau levels.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, et al. (1995) Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience 69: 869–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH091037/MH/NIMH NIH HHS/United States
- R01 GM054051/GM/NIGMS NIH HHS/United States
- AG047781/AG/NIA NIH HHS/United States
- DA024860/DA/NIDA NIH HHS/United States
- R01 AG047781/AG/NIA NIH HHS/United States
- GM054051/GM/NIGMS NIH HHS/United States
- MH095532/MH/NIMH NIH HHS/United States
- MH52711/MH/NIMH NIH HHS/United States
- NS04933901/NS/NINDS NIH HHS/United States
- R03 MH095532/MH/NIMH NIH HHS/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- PL1 DA024860/DA/NIDA NIH HHS/United States
- R01 MH052711/MH/NIMH NIH HHS/United States
- R01 MH091037/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

