Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism
- PMID: 25093613
- PMCID: PMC4314516
- DOI: 10.1016/j.bbalip.2014.07.020
Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism
Abstract
Polyunsaturated fatty acids (PUFA) are oxidized by cytochrome P450 epoxygenases to PUFA epoxides which function as potent lipid mediators. The major metabolic pathways of PUFA epoxides are incorporation into phospholipids and hydrolysis to the corresponding PUFA diols by soluble epoxide hydrolase. Inhibitors of soluble epoxide hydrolase stabilize PUFA epoxides and potentiate their functional effects. The epoxyeicosatrienoic acids (EETs) synthesized from arachidonic acid produce vasodilation, stimulate angiogenesis, have anti-inflammatory actions, and protect the heart against ischemia-reperfusion injury. EETs produce these functional effects by activating receptor-mediated signaling pathways and ion channels. The epoxyeicosatetraenoic acids synthesized from eicosapentaenoic acid and epoxydocosapentaenoic acids synthesized from docosahexaenoic acid are potent inhibitors of cardiac arrhythmias. Epoxydocosapentaenoic acids also inhibit angiogenesis, decrease inflammatory and neuropathic pain, and reduce tumor metastasis. These findings indicate that a number of the beneficial functions of PUFA may be due to their conversion to PUFA epoxides. This article is part of a Special Issue entitled "Oxygenated metabolism of PUFA: analysis and biological relevance".
Keywords: Arachidonic acid (AA); Docosahexaenoic acid (DHA); Eicosapentaenoic acid (EPA); Epoxydocosapentaenoic acid (EpDPE); Epoxyeicosatetraenoic acid (EpETE); Epoxyeicosatrienoic acid (EET).
Published by Elsevier B.V.
Figures

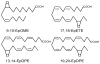

References
-
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of arachidonic acid monooxygenases. J Lipid Res. 2000;41:163–181. - PubMed
-
- Oliw EH. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Prog Lipid Res. 1994;33:329–354. - PubMed
-
- Roman R. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. - PubMed
-
- Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

