Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio)
- PMID: 25093675
- PMCID: PMC4122407
- DOI: 10.1371/journal.pone.0104330
Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio)
Abstract
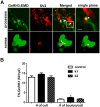
Kisspeptin1 (product of the Kiss1 gene) is the key neuropeptide that gates puberty and maintains fertility by regulating the gonadotropin-releasing hormone (GnRH) neuronal system in mammals. Inactivating mutations in Kiss1 and the kisspeptin receptor (GPR54/Kiss1r) are associated with pubertal failure and infertility. Kiss2, a paralogous gene for kiss1, has been recently identified in several vertebrates including zebrafish. Using our transgenic zebrafish model system in which the GnRH3 promoter drives expression of emerald green fluorescent protein, we investigated the effects of kisspeptins on development of the GnRH neuronal system during embryogenesis and on electrical activity during adulthood. Quantitative PCR showed detectable levels of kiss1 and kiss2 mRNA by 1 day post fertilization, increasing throughout embryonic and larval development. Early treatment with Kiss1 or Kiss2 showed that both kisspeptins stimulated proliferation of trigeminal GnRH3 neurons located in the peripheral nervous system. However, only Kiss1, but not Kiss2, stimulated proliferation of terminal nerve and hypothalamic populations of GnRH3 neurons in the central nervous system. Immunohistochemical analysis of synaptic vesicle protein 2 suggested that Kiss1, but not Kiss2, increased synaptic contacts on the cell body and along the terminal nerve-GnRH3 neuronal processes during embryogenesis. In intact brain of adult zebrafish, whole-cell patch clamp recordings of GnRH3 neurons from the preoptic area and hypothalamus revealed opposite effects of Kiss1 and Kiss2 on spontaneous action potential firing frequency and membrane potential. Kiss1 increased spike frequency and depolarized membrane potential, whereas Kiss2 suppressed spike frequency and hyperpolarized membrane potential. We conclude that in zebrafish, Kiss1 is the primary stimulator of GnRH3 neuronal development in the embryo and an activator of stimulating hypophysiotropic neuron activities in the adult, while Kiss2 plays an additional role in stimulating embryonic development of the trigeminal neuronal population, but is an RFamide that inhibits electrical activity of hypophysiotropic GnRH3 neurons in the adult.
Conflict of interest statement
Figures




References
-
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, et al. (2003) The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312: 1357–1363. - PubMed
-
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, et al. (2003) The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627. - PubMed
-
- Oka Y (2009) Three types of gonadotrophin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. J Neuroendocrinol 21: 334–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

