Cellular immune response against firefly luciferase after sleeping beauty-mediated gene transfer in vivo
- PMID: 25093708
- PMCID: PMC4251089
- DOI: 10.1089/hum.2014.048
Cellular immune response against firefly luciferase after sleeping beauty-mediated gene transfer in vivo
Abstract
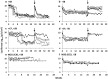
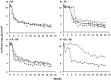
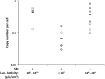
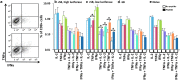
The Sleeping Beauty (SB) transposon system has been shown to mediate new gene sequence integration resulting in long-term expression. Here the effectiveness of hyperactive SB100X transposase was tested, and we found that hydrodynamic co-delivery of a firefly luciferase transposon (pT2/CaL) along with SB100X transposase (pCMV-SB100X) resulted in remarkably sustained, high levels of luciferase expression. However, after 4 weeks there was a rapid, animal-by-animal loss of luciferase expression that was not observed in immunodeficient mice. We hypothesized that this sustained, high-level luciferase expression achieved using the SB100X transposase elicits an immune response in pT2/CaL co-administered mice, which was supported by the rapid loss of luciferase expression upon challenge of previously treated animals and in naive animals adoptively transferred with splenocytes from previously treated animals. Specificity of the immune response to luciferase was demonstrated by increased cytokine expression in splenocytes after exposure to luciferase peptide in parallel with MHC I-luciferase peptide tetramer binding. This anti-luciferase immune response observed following continuous, high-level luciferase expression in vivo clearly impacts its use as an in vivo reporter. As both an immunogen and an extremely sensitive reporter, luciferase is also a useful model system for the study of immune responses following in vivo gene transfer and expression.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

