Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury
- PMID: 25093762
- PMCID: PMC4298751
- DOI: 10.1089/neu.2014.3464
Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury
Abstract
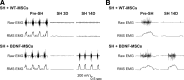
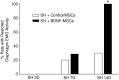
Neurotrophins, such as brain-derived neurotrophic factor (BDNF), are important in modulating neuroplasticity and promoting recovery after spinal cord injury. Intrathecal delivery of BDNF enhances functional recovery following unilateral spinal cord hemisection (SH) at C2, a well-established model of incomplete cervical spinal cord injury. We hypothesized that localized delivery of BDNF-expressing mesenchymal stem cells (BDNF-MSCs) would promote functional recovery of rhythmic diaphragm activity after SH. In adult rats, bilateral diaphragm electromyographic (EMG) activity was chronically monitored to determine evidence of complete SH at 3 days post-injury, and recovery of rhythmic ipsilateral diaphragm EMG activity over time post-SH. Wild-type, bone marrow-derived MSCs (WT-MSCs) or BDNF-MSCs (2×10(5) cells) were injected intraspinally at C2 at the time of injury. At 14 days post-SH, green fluorescent protein (GFP) immunoreactivity confirmed MSCs presence in the cervical spinal cord. Functional recovery in SH animals injected with WT-MSCs was not different from untreated SH controls (n=10; overall, 20% at 7 days and 30% at 14 days). In contrast, functional recovery was observed in 29% and 100% of SH animals injected with BDNF-MSCs at 7 days and 14 days post-SH, respectively (n=7). In BDNF-MSCs treated SH animals at 14 days, root-mean-squared EMG amplitude was 63±16% of the pre-SH value compared with 12±9% in the control/WT-MSCs group. We conclude that localized delivery of BDNF-expressing MSCs enhances functional recovery of diaphragm muscle activity following cervical spinal cord injury. MSCs can be used to facilitate localized delivery of trophic factors such as BDNF in order to promote neuroplasticity following spinal cord injury.
Keywords: diaphragm muscle; neuroplasticity; neurotrophin; respiration; spinal hemisection.
Figures


Similar articles
-
Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury.Exp Neurol. 2013 Sep;247:101-9. doi: 10.1016/j.expneurol.2013.04.002. Epub 2013 Apr 10. Exp Neurol. 2013. PMID: 23583688 Free PMC article.
-
BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury.J Neurophysiol. 2017 Feb 1;117(2):537-544. doi: 10.1152/jn.00654.2016. Epub 2016 Nov 9. J Neurophysiol. 2017. PMID: 27832605 Free PMC article.
-
Acute intrathecal BDNF enhances functional recovery after cervical spinal cord injury in rats.J Neurophysiol. 2021 Jun 1;125(6):2158-2165. doi: 10.1152/jn.00146.2021. Epub 2021 May 5. J Neurophysiol. 2021. PMID: 33949892 Free PMC article.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
-
Role of neurotrophins in recovery of phrenic motor function following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):218-25. doi: 10.1016/j.resp.2009.08.008. Epub 2009 Aug 22. Respir Physiol Neurobiol. 2009. PMID: 19703592 Free PMC article. Review.
Cited by
-
Failed reinnervation in aging skeletal muscle.Skelet Muscle. 2016 Sep 1;6(1):29. doi: 10.1186/s13395-016-0101-y. eCollection 2016. Skelet Muscle. 2016. PMID: 27588166 Free PMC article.
-
Disproportionate loss of excitatory inputs to smaller phrenic motor neurons following cervical spinal hemisection.J Physiol. 2020 Oct;598(20):4693-4711. doi: 10.1113/JP280130. Epub 2020 Aug 19. J Physiol. 2020. PMID: 32735344 Free PMC article.
-
Impact of glutamatergic and serotonergic neurotransmission on diaphragm muscle activity after cervical spinal hemisection.J Neurophysiol. 2017 Sep 1;118(3):1732-1738. doi: 10.1152/jn.00345.2017. Epub 2017 Jun 28. J Neurophysiol. 2017. PMID: 28659464 Free PMC article.
-
Plasticity in respiratory motor neurons in response to reduced synaptic inputs: A form of homeostatic plasticity in respiratory control?Exp Neurol. 2017 Jan;287(Pt 2):225-234. doi: 10.1016/j.expneurol.2016.07.012. Epub 2016 Jul 22. Exp Neurol. 2017. PMID: 27456270 Free PMC article. Review.
-
BDNF Overexpression Exhibited Bilateral Effect on Neural Behavior in SCT Mice Associated with AKT Signal Pathway.Neurochem Res. 2016 Oct;41(10):2585-2597. doi: 10.1007/s11064-016-1970-5. Epub 2016 Jun 9. Neurochem Res. 2016. PMID: 27278760
References
-
- Weishaupt N., Blesch A., and Fouad K. (2012). BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 238, 254–264 - PubMed
-
- Prakash Y.S., Mantilla C.B., Zhan W.Z., Smithson K.G., and Sieck G.C. (2000). Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 89, 563–572 - PubMed
-
- Song A., Ashwell K.W., and Tracey D.J. (2000). Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat. Embryol. (Berl.) 202, 159–177 - PubMed
-
- Goshgarian H.G., and Rafols J.A. (1984). The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J. Neurocytol. 13, 85–109 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

