Clinical imaging in regenerative medicine
- PMID: 25093889
- PMCID: PMC4164232
- DOI: 10.1038/nbt.2993
Clinical imaging in regenerative medicine
Abstract
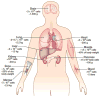
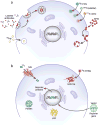
In regenerative medicine, clinical imaging is indispensable for characterizing damaged tissue and for measuring the safety and efficacy of therapy. However, the ability to track the fate and function of transplanted cells with current technologies is limited. Exogenous contrast labels such as nanoparticles give a strong signal in the short term but are unreliable long term. Genetically encoded labels are good both short- and long-term in animals, but in the human setting they raise regulatory issues related to the safety of genomic integration and potential immunogenicity of reporter proteins. Imaging studies in brain, heart and islets share a common set of challenges, including developing novel labeling approaches to improve detection thresholds and early delineation of toxicity and function. Key areas for future research include addressing safety concerns associated with genetic labels and developing methods to follow cell survival, differentiation and integration with host tissue. Imaging may bridge the gap between cell therapies and health outcomes by elucidating mechanisms of action through longitudinal monitoring.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
Figures

Comment in
-
Cardiac regeneration validated.Nat Biotechnol. 2015 Jun;33(6):587. doi: 10.1038/nbt.3254. Nat Biotechnol. 2015. PMID: 26057969 No abstract available.
-
Response to cardiac regeneration validated.Nat Biotechnol. 2015 Jun;33(6):587. doi: 10.1038/nbt.3257. Nat Biotechnol. 2015. PMID: 26057970 No abstract available.
References
-
- Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1:179–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

