Protonation of nickel-iron hydrogenase models proceeds after isomerization at nickel
- PMID: 25094041
- PMCID: PMC4156870
- DOI: 10.1021/ja505783z
Protonation of nickel-iron hydrogenase models proceeds after isomerization at nickel
Abstract
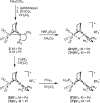
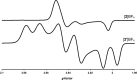
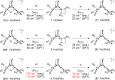
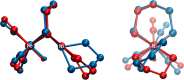
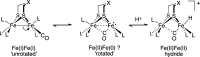
Theory and experiment indicate that the protonation of reduced NiFe dithiolates proceeds via a previously undetected isomer with enhanced basicity. In particular, it is proposed that protonation of (OC)3Fe(pdt)Ni(dppe) (1; pdt(2-) = (-)S(CH2)3S(-); dppe = Ph2P(CH2)2PPh2) occurs at the Fe site of the two-electron mixed-valence Fe(0)Ni(II) species, not the Fe(I)-Ni(I) bond for the homovalence isomer of 1. The new pathway, which may have implications for protonation of other complexes and clusters, was uncovered through studies on the homologous series L(OC)2Fe(pdt)M(dppe), where M = Ni, Pd (2), and Pt (3) and L = CO, PCy3. Similar to 1, complexes 2 and 3 undergo both protonation and 1e(-) oxidation to afford well-characterized hydrides ([2H](+) and [3H](+)) and mixed-valence derivatives ([2](+) and [3](+)), respectively. Whereas the Pd site is tetrahedral in 2, the Pt site is square-planar in 3, indicating that this complex is best described as Fe(0)Pt(II). In view of the results on 2 and 3, the potential energy surface of 1 was reinvestigated with density functional theory. These calculations revealed the existence of an energetically accessible and more basic Fe(0)Ni(II) isomer with a square-planar Ni site.
Figures






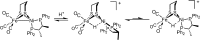

References
-
- Dyson P. J.; McIndoe J. S.. Transition Metal Carbonyl Cluster Chemistry; CRC Press: Boca Raton, FL, 2000.
- Rosenberg E.; Freeman W.; Carlos Z.; Hardcastle K.; Yoo Y. J.; Milone L.; Gobetto R. J. Cluster Sci. 1992, 3, 439.
- Hash K. R.; Rosenberg E. Organometallics 1997, 16, 3593.
-
- Kramarz K. W.; Norton J. R. Prog. Inorg. Chem. 1994, 42, 1.
-
- Lubitz W.; Ogata H.; Rüdiger O.; Reijerse E. Chem. Rev. 2014, 114, 4081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

