Chemical glycosylation of cytochrome c improves physical and chemical protein stability
- PMID: 25095792
- PMCID: PMC4137108
- DOI: 10.1186/1471-2091-15-16
Chemical glycosylation of cytochrome c improves physical and chemical protein stability
Abstract
Background: Cytochrome c (Cyt c) is an apoptosis-initiating protein when released into the cytoplasm of eukaryotic cells and therefore a possible cancer drug candidate. Although proteins have been increasingly important as pharmaceutical agents, their chemical and physical instability during production, storage, and delivery remains a problem. Chemical glycosylation has been devised as a method to increase protein stability and thus enhance their long-lasting bioavailability.
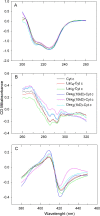
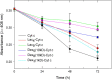
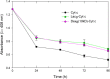
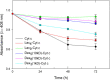
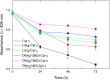
Results: Three different molecular weight glycans (lactose and two dextrans with 1 kD and 10 kD) were chemically coupled to surface exposed Cyt c lysine (Lys) residues using succinimidyl chemistry via amide bonds. Five neo-glycoconjugates were synthesized, Lac4-Cyt-c, Lac9-Cyt-c, Dex5(10kD)-Cyt-c, Dex8(10kD)-Cyt-c, and Dex3(1kD)-Cyt-c. Subsequently, we investigated glycoconjugate structure, activity, and stability. Circular dichroism (CD) spectra demonstrated that Cyt c glycosylation did not cause significant changes to the secondary structure, while high glycosylation levels caused some minor tertiary structure perturbations. Functionality of the Cyt c glycoconjugates was determined by performing cell-free caspase 3 and caspase 9 induction assays and by measuring the peroxidase-like pseudo enzyme activity. The glycoconjugates showed ≥94% residual enzyme activity and 86 ± 3 to 95 ± 1% relative caspase 3 activation compared to non-modified Cyt c. Caspase 9 activation by the glycoconjugates was with 92 ± 7% to 96 ± 4% within the error the same as the caspase 3 activation. There were no major changes in Cyt c activity upon glycosylation. Incubation of Dex3(1 kD)-Cyt c with mercaptoethanol caused significant loss in the tertiary structure and a drop in caspase 3 and 9 activation to only 24 ± 8% and 26 ± 6%, respectively. This demonstrates that tertiary structure intactness of Cyt c was essential for apoptosis induction. Furthermore, glycosylation protected Cyt c from detrimental effects by some stresses (i.e., elevated temperature and humidity) and from proteolytic degradation. In addition, non-modified Cyt c was more susceptible to denaturation by a water-organic solvent interface than its glycoconjugates, important for the formulation in polymers.
Conclusion: The results demonstrate that chemical glycosylation is a potentially valuable method to increase Cyt c stability during formulation and storage and potentially during its application after administration.
Figures

Similar articles
-
Delivery of chemically glycosylated cytochrome c immobilized in mesoporous silica nanoparticles induces apoptosis in HeLa cancer cells.Mol Pharm. 2014 Jan 6;11(1):102-11. doi: 10.1021/mp400400j. Epub 2013 Dec 10. Mol Pharm. 2014. PMID: 24294910 Free PMC article.
-
Moisture-induced solid state instabilities in alpha-chymotrypsin and their reduction through chemical glycosylation.BMC Biotechnol. 2010 Aug 9;10:57. doi: 10.1186/1472-6750-10-57. BMC Biotechnol. 2010. PMID: 20696067 Free PMC article.
-
Differential sensitivity to apoptosome apparatus activation in non-small cell lung carcinoma and the lung.Int J Oncol. 2014 May;44(5):1443-54. doi: 10.3892/ijo.2014.2333. Epub 2014 Mar 10. Int J Oncol. 2014. PMID: 24626292 Free PMC article.
-
Mechanisms of cytochrome c release from mitochondria.Cell Death Differ. 2006 Sep;13(9):1423-33. doi: 10.1038/sj.cdd.4401950. Epub 2006 May 5. Cell Death Differ. 2006. PMID: 16676004 Review.
-
The role of key residues in structure, function, and stability of cytochrome-c.Cell Mol Life Sci. 2014 Jan;71(2):229-55. doi: 10.1007/s00018-013-1341-1. Epub 2013 Apr 25. Cell Mol Life Sci. 2014. PMID: 23615770 Free PMC article. Review.
Cited by
-
Cardiac Tyrosine 97 Phosphorylation of Cytochrome c Regulates Respiration and Apoptosis.Int J Mol Sci. 2025 Feb 4;26(3):1314. doi: 10.3390/ijms26031314. Int J Mol Sci. 2025. PMID: 39941082 Free PMC article.
-
Post-Translational Modifications of Cytochrome c in Cell Life and Disease.Int J Mol Sci. 2020 Nov 11;21(22):8483. doi: 10.3390/ijms21228483. Int J Mol Sci. 2020. PMID: 33187249 Free PMC article. Review.
-
Diverse functions of cytochrome c in cell death and disease.Cell Death Differ. 2024 Apr;31(4):387-404. doi: 10.1038/s41418-024-01284-8. Epub 2024 Mar 23. Cell Death Differ. 2024. PMID: 38521844 Free PMC article. Review.
-
Smart Release Nano-formulation of Cytochrome C and Hyaluronic Acid Induces Apoptosis in Cancer Cells.J Nanomed Nanotechnol. 2017 Feb;8(1):427. doi: 10.4172/2157-7439.1000427. Epub 2017 Feb 24. J Nanomed Nanotechnol. 2017. PMID: 28706754 Free PMC article.
-
Anticancer Nanoparticle Carriers of the Proapoptotic Protein Cytochrome c.Pharmaceutics. 2025 Feb 26;17(3):305. doi: 10.3390/pharmaceutics17030305. Pharmaceutics. 2025. PMID: 40142969 Free PMC article. Review.
References
-
- Eklund JW, Trifilio S, Mulcahy MF. Chemotherapy dosing in the setting of liver dysfunction. Oncology. 2005;19:1057–1069. - PubMed
-
- Fan L, Wu H, Zhang H, Li F, Yang TH, Gu CH, Yang Q. Novel super pH-sensitive nanoparticles responsive to tumor extracellular pH. Carbohyd Polym. 2008;73:390–400.
-
- Azarmi S, Roa WH, Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 2008;60:863–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

