Phenotypic characterization of virological failure following lopinavir/ritonavir monotherapy using full-length Gag-protease genes
- PMID: 25096075
- PMCID: PMC4228778
- DOI: 10.1093/jac/dku296
Phenotypic characterization of virological failure following lopinavir/ritonavir monotherapy using full-length Gag-protease genes
Abstract
Objectives: Major protease mutations are rarely observed following first-line failure with PIs and interpretation of genotyping results in this context may be difficult. We performed extensive phenotyping of viruses from five patients failing lopinavir/ritonavir monotherapy in the MONARK study without major PI mutations by standard genotyping.
Methods: Phenotypic susceptibility testing and viral infectivity assessments were performed using a single-cycle assay and fold changes (FC) relative to a lopinavir-susceptible reference strain were calculated.
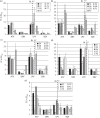
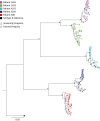
Results: >10-fold reduced baseline susceptibility to lopinavir occurred in two of five patients and >5-fold in another two. Four of five patients exhibited phylogenetic evidence of a limited viral evolution between baseline and failure, with amino acid changes at drug resistance-associated positions in one: T81A emerged in Gag with M36I in the protease gene, correlating with a reduction in lopinavir susceptibility from FC 7 (95% CI 6-8.35) to FC 13 (95% CI 8.11-17.8). Reductions in darunavir susceptibility (>5 FC) occurred in three individuals.
Discussion: This study suggests both baseline reduced susceptibility and evolution of resistance could be contributing factors to PI failure, despite the absence of classical PI resistance mutations by standard testing methods. Use of phenotyping also reveals lower darunavir susceptibility, warranting further study as this agent is commonly used following lopinavir failure.
Keywords: Gag; HIV; antiretroviral resistance; monotherapy; protease inhibitors.
© The Author 2014. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures

References
-
- Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:409–17. - PubMed
-
- Sungkanuparph S, Manosuthi W, Kiertiburanakul S, et al. Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis. 2007;44:447–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

