The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex
- PMID: 25096783
- PMCID: PMC4176441
- DOI: 10.1105/tpc.114.123455
The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex
Abstract
Potassium (K(+)) is one of the essential nutrient elements for plant growth and development. Plants absorb K(+) ions from the environment via root cell K(+) channels and/or transporters. In this study, the Shaker K(+) channel Os-AKT1 was characterized for its function in K(+) uptake in rice (Oryza sativa) roots, and its regulation by Os-CBL1 (Calcineurin B-Like protein1) and Os-CIPK23 (CBL-Interacting Protein Kinase23) was investigated. As an inward K(+) channel, Os-AKT1 could carry out K(+) uptake and rescue the low-K(+)-sensitive phenotype of Arabidopsis thaliana akt1 mutant plants. Rice Os-akt1 mutant plants showed decreased K(+) uptake and displayed an obvious low-K(+)-sensitive phenotype. Disruption of Os-AKT1 significantly reduced the K(+) content, which resulted in inhibition of plant growth and development. Similar to the AKT1 regulation in Arabidopsis, Os-CBL1 and Os-CIPK23 were identified as the upstream regulators of Os-AKT1 in rice. The Os-CBL1-Os-CIPK23 complex could enhance Os-AKT1-mediated K(+) uptake. A phenotype test confirmed that Os-CIPK23 RNAi lines exhibited similar K(+)-deficient symptoms as the Os-akt1 mutant under low K(+) conditions. These findings demonstrate that Os-AKT1-mediated K(+) uptake in rice roots is modulated by the Os-CBL1-Os-CIPK23 complex.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures
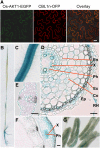

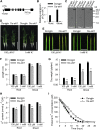
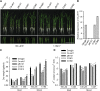

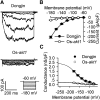

References
-
- Ashley M.K., Grant M., Grabov A. (2006). Plant responses to potassium deficiencies: a role for potassium transport proteins. J. Exp. Bot. 57: 425–436. - PubMed
-
- Boscari A., Clément M., Volkov V., Golldack D., Hybiak J., Miller A.J., Amtmann A., Fricke W. (2009). Potassium channels in barley: cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ. 32: 1761–1777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

