Protein kinase C is a calcium sensor for presynaptic short-term plasticity
- PMID: 25097249
- PMCID: PMC5841930
- DOI: 10.7554/eLife.03011
Protein kinase C is a calcium sensor for presynaptic short-term plasticity
Retraction in
-
Retraction: Protein kinase C is a calcium sensor for presynaptic short-term plasticity.Elife. 2018 Mar 7;7:e35974. doi: 10.7554/eLife.35974. Elife. 2018. PMID: 29512487 Free PMC article. No abstract available.
Abstract
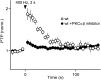
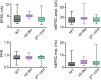
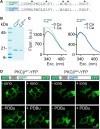
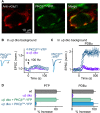
In presynaptic boutons, calcium (Ca(2+)) triggers both neurotransmitter release and short-term synaptic plasticity. Whereas synaptotagmins are known to mediate vesicle fusion through binding of high local Ca(2+) to their C2 domains, the proteins that sense smaller global Ca(2+) increases to produce short-term plasticity have remained elusive. Here, we identify a Ca(2+) sensor for post-tetanic potentiation (PTP), a form of plasticity thought to underlie short-term memory. We find that at the functionally mature calyx of Held synapse the Ca(2+)-dependent protein kinase C isoforms α and β are necessary for PTP, and the expression of PKCβ in PKCαβ double knockout mice rescues PTP. Disruption of Ca(2+) binding to the PKCβ C2 domain specifically prevents PTP without impairing other PKCβ-dependent forms of synaptic enhancement. We conclude that different C2-domain-containing presynaptic proteins are engaged by different Ca(2+) signals, and that Ca(2+) increases evoked by tetanic stimulation are sensed by PKCβ to produce PTP.DOI: http://dx.doi.org/10.7554/eLife.03011.001.
Keywords: calcium; phorbol ester; post-tetanic potentiation; protein kinase C; short-term plasticity; synaptotagmin.
Copyright © 2014, Fioravante et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

