New strategies with anti-IgE in allergic diseases
- PMID: 25097719
- PMCID: PMC4114087
- DOI: 10.1186/1939-4551-7-17
New strategies with anti-IgE in allergic diseases
Abstract
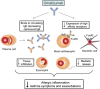
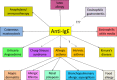
IgE has long been known as a therapeutic target for allergic disease, but the difficulty has been in selecting agents that don't trigger cross linkage of IgE when bound to its high affinity receptor (FceR1) on mast cells and basophils. By "designing" a monoclonal antibody (mAb) which targets that part of IgE that binds to that binds to the a-chain of FceR1, the allergic cascade can be effectively interrupted and diseases such as asthma greatly improved, providing a substantial part of their phenotype engages IgE. Clinical trials and real life studies confirm this. Beyond asthma, a whole range of other diseases dependent upon IgE initiation and triggering are being identified. These diseases are now being explored as being amenable to anti-IgE therapy some of which are comorbidities of asthma and others not. The advent of an even more potent anti-IgE mAb - QGE031 - is creating further opportunities for anti-IgE therapy to improve the lives of so many people with IgE-related diseases.
Keywords: Allergy; Anti-IgE monoclonal antibody; Asthma; Comorbidity; Omalizumab.
Figures
References
-
- Ishizaka K, Ishizaka T, Hornbrook M. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity and E-globulin antibody. J Immunol. 1966;7:840–853. - PubMed
-
- Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;7(2 Suppl):S65–S71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

