A prospective study comparing the efficacy and safety of two sublingual birch allergen preparations
- PMID: 25097754
- PMCID: PMC4122029
- DOI: 10.1186/2045-7022-4-23
A prospective study comparing the efficacy and safety of two sublingual birch allergen preparations
Abstract
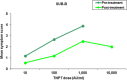
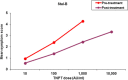
Background: SUBLIVAC FIX Birch (SUB-B) is a liquid oral preparation of Betula verrucosa pollen extract for the treatment of allergic rhinitis/rhinoconjuctivitis induced by birch pollen. The major allergen content of SUB-B and Staloral Birch (Stal-B) have been shown to be comparable. In order to compare the clinical efficacy and safety of both products, the present study was designed to investigate efficacy of treatment with SUB-B compared to Stal-B by means of reduction in allergy symptoms assessed by a titrated nasal provocation test (TNPT) in subjects suffering from IgE mediated allergy complaints triggered by birch pollen.
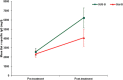
Methods: A prospective, randomized, open, blinded endpoint (PROBE), controlled, single-centre study in 74 birch allergic adults was performed. Treatment consisted of either SUB-B (10,000 AUN/ml) or Stal-B (initial phase 10 I.R./ml and maintenance phase 300 I.R./ml) for 16-20 weeks at maintenance dose. The primary efficacy outcome was defined by the difference in change of the TNPT-threshold dose between the two treatment groups at baseline and after completion of treatment. Secondary outcomes included determination of birch pollen specific IgE and IgG levels, safety lab and ECG. During the first 30 days of treatment, subjects were requested to fill out a diary concerning compliance with study medication, occurrence of AEs and the use of concomitant medication.
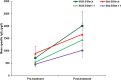
Results: Analysis of the primary efficacy parameter showed that the percentage of subjects showing a beneficial treatment effect was similar in both treatment groups, 33.3% for SUB-B vs. 31.4% for Stal-B in the intention to treat population. Evaluation of the immunologic response, showed that treatment with SUB-B and Stal-B induced similar increases (approximately 2 times) in IgE, IgG and IgG4 specific for Bet v 1. In total, 143 related adverse events (AEs) were reported. The majority of the AEs was of mild intensity. The same pattern of AEs was observed for both products. No clinically relevant changes in other safety parameters, such as safety laboratory parameters, vital signs, physical examination and ECGs were observed.
Conclusion: Taken together, treatment with both products was effective by means of reduction in allergic symptoms during a TNPT. In addition, safety analysis revealed a good tolerability of both SLIT extracts.
Keywords: Birch pollen allergy; Nasal provocation test (NPT); Non-inferiority design; Randomized; Sublingual immunotherapy (SLIT).
Figures

References
-
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, Van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, Van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 Update (in collaboration with the World Health Organization, GA2LEN and AllerGen) Allergy. 2008;2008(63):S8–S160. - PubMed
-
- Calderon MA, Demoly P, Gerth Van Wijk R, Bousquet J, Sheikh A, Frew A, Scadding G, Bachert C, Malling HJ, Valenta R, Bilo B, Nieto A, Akdis C, Just J, Vidal C, Varga EM, Al Varez-Cuesta E, Bohle B, Bufe A, Canonica WG, Cardona V, Dahl R, Didier A, Durham SR, Eng P, Fernandez-Rivas M, Jacobsen L, Jutel M, Kleine-Tebbe J, Klimek L. et al.EAACI: A European Declaration on Immunotherapy. Designing the future of allergen specific immunotherapy. Clin Transl Allergy. 2012;2(1):20. doi: 10.1186/2045-7022-2-20. - DOI - PMC - PubMed
-
- Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, Burastero SE, Calori G, Benetti L, Bonazza P, Puccinelli P, Parmiani S, Bernardini R, Vierucci A. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114(4):851–857. doi: 10.1016/j.jaci.2004.07.012. - DOI - PubMed
-
- Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivikko A, Norberg LA, Valovirta E, Wahn U, Möller C. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–948. doi: 10.1111/j.1398-9995.2007.01451.x. - DOI - PubMed
-
- Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, Helms PJ, Hunt J, Liu A, Papadopoulos N, Platts-Mills T, Pohunek P, Simons FE, Valovirta E, Wahn U, Wildhaber J. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

