The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection
- PMID: 25098291
- PMCID: PMC4170189
- DOI: 10.4049/jimmunol.1400728
The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection
Abstract
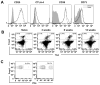
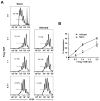
Leishmania (Viannia) parasites are etiological agents of cutaneous leishmaniasis in the New World. Infection is characterized by a mixed Th1/Th2 inflammatory response, which contributes to disease pathology. However, the role of regulatory T cells (Tregs) in Leishmania (Viannia) disease pathogenesis is unclear. Using the mouse model of chronic L. (V.) panamensis infection, we examined the hypothesis that Treg functionality contributes to control of pathogenesis. Upon infection, Tregs (CD4(+)Foxp3(+)) presented with a dysregulated phenotype, in that they produced IFN-γ, expressed Tbet, and had a reduced ability to suppress T cell proliferation in vitro. Targeted ablation of Tregs resulted in enlarged lesions, increased parasite load, and enhanced production of IL-17 and IFN-γ, with no change in IL-10 and IL-13 levels. This indicated that an increased inflammatory response was commensurate with disease exacerbation and that the remaining impaired Tregs were important in regulation of disease pathology. Conversely, adoptive transfer of Tregs from naive mice halted disease progression, lowered parasite burden, and reduced cytokine production (IL-10, IL-13, IL-17, IFN-γ). Because Tregs appeared to be important for controlling infection, we hypothesized that their expansion could be used as an immunotherapeutic treatment approach. As a proof of principle, chronically infected mice were treated with rIL-2/anti-IL-2 Ab complex to expand Tregs. Treatment transitorily increased the numbers and percentage of Tregs (draining lymph node, spleen), which resulted in reduced cytokine responses, ameliorated lesions, and reduced parasite load (10(5)-fold). Thus, immunotherapy targeting Tregs could provide an alternate treatment strategy for leishmaniasis caused by Leishmania (Viannia) parasites.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
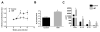

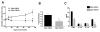
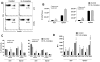
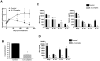
References
-
- Banuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol. 2007;64:1–109. - PubMed
-
- Lipoldova M, Demant P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet. 2006;7:294–305. - PubMed
-
- Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. - PubMed
-
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

