Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity
- PMID: 25098555
- PMCID: PMC4143994
- DOI: 10.1038/ncomms5593
Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity
Abstract
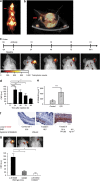
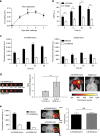
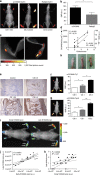
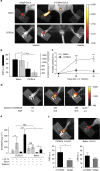
Inflammation has a key role in the pathogenesis of various human diseases. The early detection, localization and monitoring of inflammation are crucial for tailoring individual therapies. However, reliable biomarkers to detect local inflammatory activities and to predict disease outcome are still missing. Alarmins, which are locally released during cellular stress, are early amplifiers of inflammation. Here, using optical molecular imaging, we demonstrate that the alarmin S100A8/S100A9 serves as a sensitive local and systemic marker for the detection of even sub-clinical disease activity in inflammatory and immunological processes like irritative and allergic contact dermatitis. In a model of collagen-induced arthritis, we use S100A8/S100A9 imaging to predict the development of disease activity. Furthermore, S100A8/S100A9 can act as a very early and sensitive biomarker in experimental leishmaniasis for phagocyte activation linked to an effective Th1-response. In conclusion, the alarmin S100A8/S100A9 is a valuable and sensitive molecular target for novel imaging approaches to monitor clinically relevant inflammatory disorders on a molecular level.
Figures
References
-
- Chan A. C. & Behrens T. W. Personalizing medicine for autoimmune and inflammatory diseases. Nat. Immunol. 14, 106–109 (2013). - PubMed
-
- Gotthardt M., Bleeker-Rovers C. P., Boerman O. C. & Oyen W. J. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. 51, 1937–1949 (2010). - PubMed
-
- Harris H. E., Andersson U. & Pisetsky D. S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol 8, 195–202 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

