Toll-like receptor 3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway
- PMID: 25098814
- PMCID: PMC4238881
- DOI: 10.1111/cei.12432
Toll-like receptor 3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway
Abstract
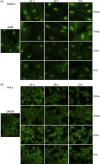
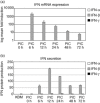
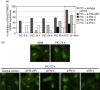
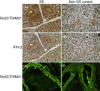
Up-regulated expression of Ro52/tripartite motif-containing protein 21 (TRIM21), Ro60/TROVE domain family, member 2 (TROVE2) and lupus LA protein/Sjögren's syndrome antigen B (La/SSB) autoantigens has been described in the salivary gland epithelial cells (SGEC) of patients with Sjögren's syndrome (SS). SGECs, the key regulators of autoimmune SS responses, express high levels of surface functional Toll-like receptor (TLR)-3, whereas Ro52/TRIM21 negatively regulates TLR-3-mediated inflammation. Herein, we investigated the effect of TLR-3-signalling on the expression of Ro52/TRIM21, as well as Ro60/TROVE2 and La/SSB autoantigens, by SGECs. The effect of TLR-3 or TLR-4 stimulation on autoantigen expression was evaluated by polyI:C or lipopolysaccharide (LPS) treatment, respectively, of SGEC lines (10 from SS patients, 12 from non-SS controls) or HeLa cells, followed by analysis of mRNA and protein expression. PolyI:C, but not LPS, resulted in a two-step induction of Ro52/TRIM21 mRNA expression by SGECs, a 12-fold increment at 6 h followed by a 2.5-fold increment at 24-48 h, whereas it induced a late two-fold up-regulation of Ro60/TROVE2 and La/SSB mRNAs at 48 h. Although protein expression levels were not affected significantly, the late up-regulation of Ro52/TRIM21 mRNA was accompanied by protein redistribution, from nucleolar-like pattern to multiple coarse dots spanning throughout the nucleus. These late phenomena were mediated significantly by interferon (IFN)-β production, as attested by cognate secretion and specific inhibition experiments and associated with IFN regulatory factor (IRF)3 degradation. TLR-3-signalling had similar effects on SGECs obtained from SS patients and controls, whereas it did not affect the expression of these autoantigens in HeLa cells. TLR-3 signalling regulates the expression of autoantigens by SGECs, implicating innate immunity pathways in their over-expression in inflamed tissues and possibly in their exposure to the immune system.
Keywords: Ro52/TRIM21 autoantigen; Sjögren's syndrome; TLR-3; salivary gland epithelial cells; type I interferons.
© 2014 British Society for Immunology.
Figures



References
-
- Routsias JG, Vlachoyiannopoulos PG, Tzioufas AG. Autoantibodies to intracellular autoantigens and their B-cell epitopes: molecular probes to study the autoimmune response. Crit Rev Clin Lab Sci. 2006;43:203–248. - PubMed
-
- Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren's syndrome. Arthritis Rheum. 1998;41:2238–2248. - PubMed
-
- Tzioufas AG, Hantoumi I, Polihronis M, Xanthou G, Moutsopoulos HM. Autoantibodies to La/SSB in patients with primary Sjogren's syndrome (pSS) are associated with upregulation of La/SSB mRNA in minor salivary gland biopsies (MSGs) J Autoimmun. 1999;13:429–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

