Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing
- PMID: 25099164
- PMCID: PMC4206662
- DOI: 10.1038/clpt.2014.159
Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing
Abstract
Phenytoin is a widely used antiepileptic drug with a narrow therapeutic index and large interpatient variability, partly due to genetic variations in the gene encoding cytochrome P450 (CYP)2C9 (CYP2C9). Furthermore, the variant allele HLA-B*15:02, encoding human leukocyte antigen, is associated with an increased risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in response to phenytoin treatment. We summarize evidence from the published literature supporting these associations and provide recommendations for the use of phenytoin based on CYP2C9 and/or HLA-B genotype (also available on PharmGKB: http://www.pharmgkb.org). The purpose of this guideline is to provide information for the interpretation of HLA-B and/or CYP2C9 genotype tests so that the results can guide dosing and/or use of phenytoin. Detailed guidelines for the use of phenytoin as well as analyses of cost-effectiveness are out of scope. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are periodically updated at http://www.pharmgkb.org.
Figures
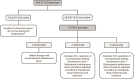
References
-
- Martin, M.A. , Klein, T.E. , Dong, B.J. , Pirmohamed, M. , Haas, D.W. & Kroetz, D.L. ; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for HLA‐B genotype and abacavir dosing. Clin. Pharmacol. Ther. 91, 734–738 (2012). - PMC - PubMed
-
- Lee, C.R. , Goldstein, J.A. & Pieper, J.A. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in‐vitro and human data. Pharmacogenetics 12, 251–263 (2002). - PubMed
-
- Twardowschy, C.A. , Werneck, L.C. , Scola, R.H. , Borgio, J.G. , De Paola, L. & Silvado, C. The role of CYP2C9 polymorphisms in phenytoin‐related cerebellar atrophy. Seizure 22, 194–197 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

