Comparative pharmacogenetic analysis of risk polymorphisms in Caucasian and Vietnamese children with acute lymphoblastic leukemia: prediction of therapeutic outcome?
- PMID: 25099492
- PMCID: PMC4345953
- DOI: 10.1111/bcp.12481
Comparative pharmacogenetic analysis of risk polymorphisms in Caucasian and Vietnamese children with acute lymphoblastic leukemia: prediction of therapeutic outcome?
Abstract
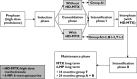
Aims: Acute lymphoblastic leukemia (ALL) is the most common of all paediatric cancers. Aside from predisposing to ALL, polymorphisms could also be associated with poor outcome. Indeed, genetic variations involved in drug metabolism could, at least partially, be responsible for heterogeneous responses to standardized leukemia treatments, hence requiring more personalized therapy. The aims of this study were to (a) to determine the prevalence of seven common genetic polymorphisms including those that affect the folate and/or thiopurine metabolic pathways, i.e. cyclin D1 (CCND1-G870A), γ-glutamyl hydrolase (GGH-C452T), methylenetetrahydrofolate reductase (MTHFR-C677T and MTHFR-A1298C), thymidylate synthase promoter (TYMS-TSER), thiopurine methyltransferase (TPMT*3A and TPMT*3C) and inosine triphosphate pyrophosphatase (ITPA-C94A), in Caucasian (n = 94, age < 20) and Vietnamese (n = 141, age < 16 years) childhood ALL and (b) to assess the impact of a multilocus genetic risk score (MGRS) on relapse-free survival (RFS) using a Cox proportional-hazards regression model.
Results: The prevalence of MTHFR-677TT genotype was significantly higher in Caucasians (P = 0.008), in contrast to the prevalence of TYMS-TSER*3R/3R and ITPA-94AA/AC genotypes which were significantly higher in Vietnamese (P < 0.001 and P = 0.02, respectively). Compared with children with a low MGRS (≤ 3), those with a high MGRS (≥ 4) were 2.06 (95% CI = 1.01, 4.22; P = 0.04) times more likely to relapse. Adding MGRS into a multivariate Cox regression model with race/ethnicity and four clinical variables improved the predictive accuracy of the model (AUC from 0.682 to 0.709 at 24 months).
Conclusion: Including MGRS into a clinical model improved the predictive accuracy of short and medium term prognosis, hence confirming the association between well determined pharmacogenotypes and outcome of paediatric ALL. Whether variants on other genes associated with folate metabolism can substantially improve the predictive value of current MGRS is not known but deserves further evaluation.
Keywords: Caucasian; Vietnamese; leukemia; pharmacogenetics; single nucleotide polymorphisms.
© 2014 The British Pharmacological Society.
Figures
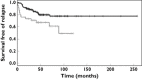
 , multilocus genetic risk score <=3;
, multilocus genetic risk score <=3;  , multilocus genetic risk score >=4
, multilocus genetic risk score >=4
 , Cox reression equation including the indicator of a multilocus genetic risk score >=4;
, Cox reression equation including the indicator of a multilocus genetic risk score >=4;  , Cox reression equation without the indicator of a multilocus genetic risk score >=4
, Cox reression equation without the indicator of a multilocus genetic risk score >=4References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Cheok MH, Evans WE. Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nat Rev Cancer. 2006;6:117–129. - PubMed
-
- Kager L, Evans WE. Pharmacogenomics of acute lymphoblastic leukemia. Curr Opin Hematol. 2006;13:260–265. - PubMed
-
- Adam de Beaumais T, Jacqz-Aigrain E. Pharmacogenetic determinants of mercaptopurine disposition in children with acute lymphoblastic leukemia. Eur J Clin Pharmacol. 2012;68:1233–1242. - PubMed
-
- Evans WE, Relling MV. Moving towards individualized medicine with pharmacogenomics. Nature. 2004;429:464–468. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

