Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST)
- PMID: 25099655
- PMCID: PMC4161926
- DOI: 10.1245/s10434-014-3908-y
Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST)
Abstract
Purpose: The purpose of the NBRST study is to compare a multigene classifier to conventional immunohistochemistry (IHC)/fluorescence in situ hybridization (FISH) subtyping to predict chemosensitivity as defined by pathological complete response (pCR) or endocrine sensitivity as defined by partial response.
Methods: The study includes women with histologically proven breast cancer, who will receive neoadjuvant chemotherapy (NCT) or neoadjuvant endocrine therapy. BluePrint in combination with MammaPrint classifies patients into four molecular subgroups: Luminal A, Luminal B, HER2, and Basal.
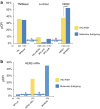
Results: A total of 426 patients had definitive surgery. Thirty-seven of 211 (18 %) IHC/FISH hormone receptor (HR)+/HER2- patients were reclassified by Blueprint as Basal (n = 35) or HER2 (n = 2). Fifty-three of 123 (43 %) IHC/FISH HER2+ patients were reclassified as Luminal (n = 36) or Basal (n = 17). Four of 92 (4 %) IHC/FISH triple-negative (TN) patients were reclassified as Luminal (n = 2) or HER2 (n = 2). NCT pCR rates were 2 % in Luminal A and 7 % Luminal B patients versus 10 % pCR in IHC/FISH HR+/HER2- patients. The NCT pCR rate was 53 % in BluePrint HER2 patients. This is significantly superior (p = 0.047) to the pCR rate in IHC/FISH HER2+ patients (38 %). The pCR rate of 36 of 75 IHC/FISH HER2+/HR+ patients reclassified as BPLuminal is 3 %. NCT pCR for BluePrint Basal patients was 49 of 140 (35 %), comparable to the 34 of 92 pCR rate (37 %) in IHC/FISH TN patients.
Conclusions: BluePrint molecular subtyping reclassifies 22 % (94/426) of tumors, reassigning more responsive patients to the HER2 and Basal categories while reassigning less responsive patients to the Luminal category. These findings suggest that compared with IHC/FISH, BluePrint more accurately identifies patients likely to respond (or not respond) to NCT.
Figures
References
-
- Viale G, Slaets L, de Snoo F, et al. Pathological assessment of discordant cases for molecular (BluePrint and MammaPrint) versus clinical subtypes for breast cancer among 621 patients from the EORTC 10041/BIG 3-04 (MINDACT) trial. Cancer Res. 2012;72(24 Suppl):303s.
-
- Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi: 10.1200/JOP.777003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

