Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality
- PMID: 25099709
- PMCID: PMC4106199
- DOI: 10.1136/bmj.g4561
Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality
Erratum in
- BMJ. 2014;349:g4850
Abstract
Objective: To assess the efficacy and safety of pooled human albumin solutions as part of fluid volume expansion and resuscitation (with or without improvement of baseline hypoalbuminaemia) in critically unwell adults with sepsis of any severity.
Design: Systematic review and meta-analysis of randomised clinical trials, with trial sequential analysis, subgroup, and meta-regression analyses.
Data sources: PubMed, PubMed Central, Web of Science (includes Medline, Conference Proceedings Citation Index, Data Citation Index, Chinese Science Citation Database, CAB abstracts, Derwent Innovations Index), OvidSP (includes Embase, Ovid Medline, HMIC, PsycINFO, Maternity and Infant Care, Transport Database), Cochrane Library, clinicaltrials.gov, controlled-trials.com, online material, relevant conference proceedings, hand searching of reference lists, and contact with authors as necessary.
Eligibility criteria: Prospective randomised clinical trials of adults with sepsis of any severity (with or without baseline hypoalbuminaemia) in critical or intensive care who received pooled human albumin solutions as part of fluid volume expansion and resuscitation (with or without improvement of hypoalbuminaemia) compared with those who received control fluids (crystalloid or colloid), were included if all-cause mortality outcome data were available. No restriction of language, date, publication status, or primary study endpoint was applied.
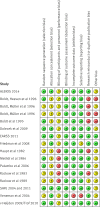
Data extraction: Two reviewers independently assessed articles for inclusion, extracted data to assess risk of bias, trial methods, patients, interventions, comparisons, and outcome. The relative risk of all-cause mortality was calculated using a random effects model accounting for clinical heterogeneity.
Primary outcome measure: All-cause mortality at final follow-up.
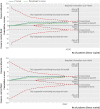
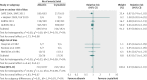
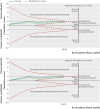
Results: Eighteen articles reporting on 16 primary clinical trials that included 4190 adults in critical or intensive care with sepsis, severe sepsis, or septic shock. A median of 70.0 g daily of pooled human albumin was received over a median of 3 days by adults with a median age of 60.8 years as part of fluid volume expansion and resuscitation, with or without correction of hypoalbuminaemia. The relative risk of death was similar between albumin groups (that received a median of 175 g in total) and control fluid groups (relative risk 0.94; 95% confidence interval 0.87 to 1.01; P=0.11; I(2)=0%). Trial sequential analysis corrected the 95% confidence interval for random error (0.85 to 1.02; D(2)=0%). Eighty eight per cent of the required information size (meta-analysis sample size) of 4894 patients was achieved, and the cumulative effect size measure (z score) entered the futility area, supporting the notion of no relative benefit of albumin (GRADE quality of evidence was moderate). Evidence of no difference was also found when albumin was compared with crystalloid fluid (relative risk 0.93; 0.86 to 1.01; P=0.07; I(2)=0%) in 3878 patents (GRADE quality of evidence was high; 79.9% of required information size) or colloid fluids in 299 patients (relative risk 1.04; 0.79 to 1.38; P=0.76; I(2)=0%) (GRADE quality of evidence was very low; 5.8% of required information size). When studies at high risk of bias were excluded in a predefined subgroup analysis, the finding of no mortality benefit remained, and the cumulative z score was just outside the boundary of futility. Overall, the meta-analysis was robust to sensitivity, subgroup, meta-regression, and trial sequential analyses.
Conclusions: In this analysis, human albumin solutions as part of fluid volume expansion and resuscitation for critically unwell adults with sepsis of any severity (with or without baseline hypoalbuminaemia) were not robustly effective at reducing all-cause mortality. Albumin seems to be safe in this setting, as a signal towards harm was not detected, but this analysis does not support a recommendation for use.
© Patel et al 2014.
Conflict of interest statement
Competing interests All authors have completed the ICMJE uniform disclosure form at
Figures



Comment in
-
Fluid resuscitation for people with sepsis.BMJ. 2014 Jul 22;349:g4611. doi: 10.1136/bmj.g4611. BMJ. 2014. PMID: 25099711 No abstract available.
References
-
- Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2013;2:CD000567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
