In situ time-resolved FRET reveals effects of sarcomere length on cardiac thin-filament activation
- PMID: 25099807
- PMCID: PMC4129473
- DOI: 10.1016/j.bpj.2014.05.044
In situ time-resolved FRET reveals effects of sarcomere length on cardiac thin-filament activation
Abstract
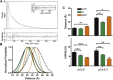
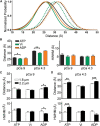
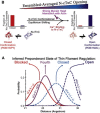
During cardiac thin-filament activation, the N-domain of cardiac troponin C (N-cTnC) binds to Ca(2+) and interacts with the actomyosin inhibitory troponin I (cTnI). The interaction between N-cTnC and cTnI stabilizes the Ca(2+)-induced opening of N-cTnC and is presumed to also destabilize cTnI-actin interactions that work together with steric effects of tropomyosin to inhibit force generation. Recently, our in situ steady-state FRET measurements based on N-cTnC opening suggested that at long sarcomere length, strongly bound cross-bridges indirectly stabilize this Ca(2+)-sensitizing N-cTnC-cTnI interaction through structural effects on tropomyosin and cTnI. However, the method previously used was unable to determine whether N-cTnC opening depends on sarcomere length. In this study, we used time-resolved FRET to monitor the effects of cross-bridge state and sarcomere length on the Ca(2+)-dependent conformational behavior of N-cTnC in skinned cardiac muscle fibers. FRET donor (AEDANS) and acceptor (DDPM)-labeled double-cysteine mutant cTnC(T13C/N51C)AEDANS-DDPM was incorporated into skinned muscle fibers to monitor N-cTnC opening. To study the structural effects of sarcomere length on N-cTnC, we monitored N-cTnC opening at relaxing and saturating levels of Ca(2+) and 1.80 and 2.2-μm sarcomere length. Mg(2+)-ADP and orthovanadate were used to examine the structural effects of noncycling strong-binding and weak-binding cross-bridges, respectively. We found that the stabilizing effect of strongly bound cross-bridges on N-cTnC opening (which we interpret as transmitted through related changes in cTnI and tropomyosin) become diminished by decreases in sarcomere length. Additionally, orthovanadate blunted the effect of sarcomere length on N-cTnC conformational behavior such that weak-binding cross-bridges had no effect on N-cTnC opening at any tested [Ca(2+)] or sarcomere length. Based on our findings, we conclude that the observed sarcomere length-dependent positive feedback regulation is a key determinant in the length-dependent Ca(2+) sensitivity of myofilament activation and consequently the mechanism underlying the Frank-Starling law of the heart.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Cross-talk, cross-bridges, and calcium activation of cardiac contraction.Biophys J. 2014 Aug 5;107(3):543-545. doi: 10.1016/j.bpj.2014.06.019. Biophys J. 2014. PMID: 25099792 Free PMC article. No abstract available.
References
-
- Tobacman L.S. Thin filament-mediated regulation of cardiac contraction. Annu. Rev. Physiol. 1996;58:447–481. - PubMed
-
- Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. - PubMed
-
- Li M.X., Spyracopoulos L., Sykes B.D. Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999;38:8289–8298. - PubMed
-
- Dong W.J., Xing J., Cheung H.C. Conformation of the regulatory domain of cardiac muscle troponin C in its complex with cardiac troponin I. J. Biol. Chem. 1999;274:31382–31390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

