DNA mismatch repair and oxidative DNA damage: implications for cancer biology and treatment
- PMID: 25099886
- PMCID: PMC4190558
- DOI: 10.3390/cancers6031597
DNA mismatch repair and oxidative DNA damage: implications for cancer biology and treatment
Abstract
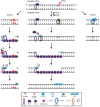
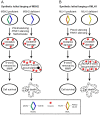
Many components of the cell, including lipids, proteins and both nuclear and mitochondrial DNA, are vulnerable to deleterious modifications caused by reactive oxygen species. If not repaired, oxidative DNA damage can lead to disease-causing mutations, such as in cancer. Base excision repair and nucleotide excision repair are the two DNA repair pathways believed to orchestrate the removal of oxidative lesions. However, recent findings suggest that the mismatch repair pathway may also be important for the response to oxidative DNA damage. This is particularly relevant in cancer where mismatch repair genes are frequently mutated or epigenetically silenced. In this review we explore how the regulation of oxidative DNA damage by mismatch repair proteins may impact on carcinogenesis. We discuss recent studies that identify potential new treatments for mismatch repair deficient tumours, which exploit this non-canonical role of mismatch repair using synthetic lethal targeting.
Figures
References
-
- Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; New York, NY, USA: 2007.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

