Single-molecule force spectroscopy reveals force-enhanced binding of calcium ions by gelsolin
- PMID: 25100107
- PMCID: PMC4143929
- DOI: 10.1038/ncomms5623
Single-molecule force spectroscopy reveals force-enhanced binding of calcium ions by gelsolin
Abstract
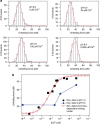
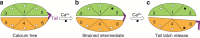
Force is increasingly recognized as an important element in controlling biological processes. Forces can deform native protein conformations leading to protein-specific effects. Protein-protein binding affinities may be decreased, or novel protein-protein interaction sites may be revealed, on mechanically stressing one or more components. Here we demonstrate that the calcium-binding affinity of the sixth domain of the actin-binding protein gelsolin (G6) can be enhanced by mechanical force. Our kinetic model suggests that the calcium-binding affinity of G6 increases exponentially with force, up to the point of G6 unfolding. This implies that gelsolin may be activated at lower calcium ion levels when subjected to tensile forces. The demonstration that cation-protein binding affinities can be force-dependent provides a new understanding of the complex behaviour of cation-regulated proteins in stressful cellular environments, such as those found in the cytoskeleton-rich leading edge and at cell adhesions.
Figures




Similar articles
-
The calcium activation of gelsolin: insights from the 3A structure of the G4-G6/actin complex.J Mol Biol. 2002 Dec 6;324(4):691-702. doi: 10.1016/s0022-2836(02)01131-2. J Mol Biol. 2002. PMID: 12460571
-
The crystal structure of the C-terminus of adseverin reveals the actin-binding interface.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13719-24. doi: 10.1073/pnas.0812383106. Epub 2009 Aug 4. Proc Natl Acad Sci U S A. 2009. PMID: 19666531 Free PMC article.
-
Helix straightening as an activation mechanism in the gelsolin superfamily of actin regulatory proteins.J Biol Chem. 2009 Aug 7;284(32):21265-9. doi: 10.1074/jbc.M109.019760. Epub 2009 Jun 1. J Biol Chem. 2009. PMID: 19491107 Free PMC article.
-
Structural aspects of actin-binding proteins.Curr Opin Cell Biol. 1994 Feb;6(1):87-95. doi: 10.1016/0955-0674(94)90121-x. Curr Opin Cell Biol. 1994. PMID: 8167031 Review.
-
Gelsolin: the tail of a molecular gymnast.Cytoskeleton (Hoboken). 2013 Jul;70(7):360-84. doi: 10.1002/cm.21117. Epub 2013 Jun 27. Cytoskeleton (Hoboken). 2013. PMID: 23749648 Review.
Cited by
-
Adaptable hydrogel networks with reversible linkages for tissue engineering.Adv Mater. 2015 Jul 1;27(25):3717-36. doi: 10.1002/adma.201501558. Epub 2015 May 19. Adv Mater. 2015. PMID: 25989348 Free PMC article. Review.
-
Mechano-adaptive sensory mechanism of α-catenin under tension.Sci Rep. 2016 Apr 25;6:24878. doi: 10.1038/srep24878. Sci Rep. 2016. PMID: 27109499 Free PMC article.
-
Functional characterization of alternatively spliced GSN in head and neck squamous cell carcinoma.Transl Res. 2018 Dec;202:109-119. doi: 10.1016/j.trsl.2018.07.007. Epub 2018 Jul 26. Transl Res. 2018. PMID: 30118659 Free PMC article.
-
Single-particle virology.Biophys Rev. 2020 Oct;12(5):1141-1154. doi: 10.1007/s12551-020-00747-9. Epub 2020 Sep 3. Biophys Rev. 2020. PMID: 32880826 Free PMC article. Review.
-
An ester bond underlies the mechanical strength of a pathogen surface protein.Nat Commun. 2021 Aug 23;12(1):5082. doi: 10.1038/s41467-021-25425-6. Nat Commun. 2021. PMID: 34426584 Free PMC article.
References
-
- Nag S., Larsson M., Robinson R. C. & Burtnick L. D. Gelsolin: the tail of a molecular gymnast. Cytoskeleton (Hoboken) 70, 360–384 (2013). - PubMed
-
- Sun H. Q., Yamamoto M., Mejillano M. & Yin H. L. Gelsolin, a multifunctional actin regulatory protein. J. Biol. Chem. 274, 33179–33182 (1999). - PubMed
-
- Ashish et al. Global structure changes associated with Ca2+ activation of full-length human plasma gelsolin. J. Biol. Chem. 282, 25884–25892 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

