Preclinical characterization of recombinant human tissue kallikrein-1 as a novel treatment for type 2 diabetes mellitus
- PMID: 25100328
- PMCID: PMC4123992
- DOI: 10.1371/journal.pone.0103981
Preclinical characterization of recombinant human tissue kallikrein-1 as a novel treatment for type 2 diabetes mellitus
Abstract
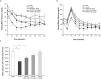
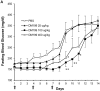
Modulation of the kallikrein-kinin system (KKS) has been shown to have beneficial effects on glucose homeostasis and several other physiological responses relevant to the progression of type 2 diabetes mellitus (T2D). The importance of bradykinin and its receptors in mediating these responses is well documented, but the role of tissue kallikrein-1, the protease that generates bradykinin in situ, is much less understood. We developed and tested DM199, recombinant human tissue kallikrein-1 protein (rhKLK-1), as a potential novel therapeutic for T2D. Hyperinsulinemic-euglycemic clamp studies suggest that DM199 increases whole body glucose disposal in non-diabetic rats. Single-dose administration of DM199 in obese db/db mice and ZDF rats, showed an acute, dose-dependent improvement in whole-body glucose utilization. Sub-acute dosing for a week in ZDF rats improved glucose utilization, with a concomitant rise in fasting insulin levels and HOMA1-%B scores. After cessation of sub-acute dosing, fasting blood glucose levels were significantly lower in ZDF rats during a drug wash-out period. Our studies show for the first time that DM199 administration results in acute anti-hyperglycemic effects in several preclinical models, and demonstrate the potential for further development of DM199 as a novel therapeutic for T2D.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

