Immunomodulatory oligonucleotides inhibit neutrophil migration by decreasing the surface expression of interleukin-8 and leukotriene B4 receptors
- PMID: 25100544
- PMCID: PMC4298415
- DOI: 10.1111/imm.12368
Immunomodulatory oligonucleotides inhibit neutrophil migration by decreasing the surface expression of interleukin-8 and leukotriene B4 receptors
Abstract
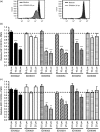
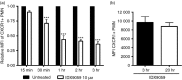
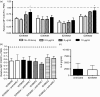
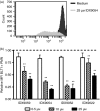
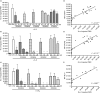
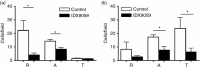
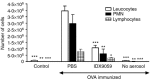
Neutrophils play important roles in many inflammatory diseases. The migration of neutrophils to the inflammatory site is tightly regulated by specific chemokines, of which interleukin-8 (IL-8) and leukotriene B4 (LTB4 ) constitute key mediators by binding to the surface receptors CXCR1/2 and BLT1, respectively. Oligonucleotides (ODN) containing CpG motifs mediate potent immunomodulatory effects through binding to Toll-like receptor 9. So far, knowledge on how ODN can affect neutrophil migration during inflammation is lacking. This study demonstrates that several novel CpG ODN significantly down-regulate the surface expression of CXCR1/2 and BLT1. In addition, the ODN significantly blocked IL-8-induced and LTB4 -induced neutrophil migration in vitro, as well as leucocyte migration in vivo demonstrated in mice by intravital microscopy and in a model of airway inflammation. The down-regulation of CXCR1 is rapid, occurring 15 min after ODN stimulation, and can be mediated through an endosomally independent mechanism. Inhibition of the IL-8 and LTB4 pathways may provide new opportunities of therapeutic intervention using ODN to reduce neutrophil infiltration during inflammation.
Keywords: chemotaxis; inflammation; neutrophils; oligonucleotide.
© 2014 John Wiley & Sons Ltd.
Figures
Similar articles
-
Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice.Br J Pharmacol. 2008 May;154(2):460-70. doi: 10.1038/bjp.2008.94. Epub 2008 Mar 24. Br J Pharmacol. 2008. PMID: 18362895 Free PMC article.
-
Leukotriene B₄-leukotriene B₄ receptor axis promotes oxazolone-induced contact dermatitis by directing skin homing of neutrophils and CD8⁺ T cells.Immunology. 2015 Sep;146(1):50-8. doi: 10.1111/imm.12478. Epub 2015 Jun 29. Immunology. 2015. PMID: 25959240 Free PMC article.
-
The leukotriene B4-leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment.Kidney Int. 2017 Jul;92(1):89-100. doi: 10.1016/j.kint.2017.01.009. Epub 2017 Mar 15. Kidney Int. 2017. PMID: 28318626
-
Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases.Curr Top Med Chem. 2006;6(13):1345-52. doi: 10.2174/15680266106061345. Curr Top Med Chem. 2006. PMID: 16918453 Review.
-
[A Research on Drug Discovery for Intracerebral Hemorrhage Focusing on Leukotriene B4 and Its Receptor].Yakugaku Zasshi. 2020;140(11):1323-1327. doi: 10.1248/yakushi.20-00145. Yakugaku Zasshi. 2020. PMID: 33132267 Review. Japanese.
Cited by
-
MHC Class II Activation and Interferon-γ Mediate the Inhibition of Neutrophils and Eosinophils by Staphylococcal Enterotoxin Type A (SEA).Front Cell Infect Microbiol. 2017 Dec 13;7:518. doi: 10.3389/fcimb.2017.00518. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29322036 Free PMC article.
-
Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient.Stem Cell Rev Rep. 2017 Oct;13(5):686-698. doi: 10.1007/s12015-017-9752-2. Stem Cell Rev Rep. 2017. PMID: 28710685
-
Nanomedicine regulating PSC-mediated intercellular crosstalk: Mechanisms and therapeutic strategies.Acta Pharm Sin B. 2024 Nov;14(11):4756-4775. doi: 10.1016/j.apsb.2024.07.007. Epub 2024 Jul 10. Acta Pharm Sin B. 2024. PMID: 39664424 Free PMC article. Review.
-
CpG-oligodeoxynucleotides challenged macrophages ameliorate acetaminophen induced liver injury by activating TLR9/IRG1/itaconate metabolic pathway.Mol Med. 2025 Aug 25;31(1):282. doi: 10.1186/s10020-025-01324-0. Mol Med. 2025. PMID: 40855408 Free PMC article.
-
Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts.Microb Biotechnol. 2016 Nov;9(6):758-771. doi: 10.1111/1751-7915.12374. Epub 2016 Jun 20. Microb Biotechnol. 2016. PMID: 27319803 Free PMC article.
References
-
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. - PubMed
-
- Wilson HL, Dar A, Napper SK, Marianela Lopez A, Babiuk LA, Mutwiri GK. Immune mechanisms and therapeutic potential of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:183–213. - PubMed
-
- Musch E, Lutfi T, von Stein P, Zargari A, Admyre C, Malek M, Lofberg R, von Stein OD. Topical treatment with the Toll-like receptor agonist DIMS0150 has potential for lasting relief of symptoms in patients with chronic active ulcerative colitis by restoring glucocorticoid sensitivity. Inflamm Bowel Dis. 2012;19:283–92. - PubMed
-
- El Kebir D, Jozsef L, Filep JG. Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch Immunol Ther Exp (Warsz) 2008;56:41–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

