Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats
- PMID: 25100595
- PMCID: PMC4200110
- DOI: 10.1523/JNEUROSCI.1060-14.2014
Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats
Abstract
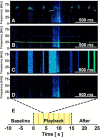
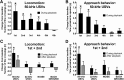
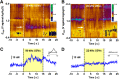
Rats emit ultrasonic vocalizations (USVs) that are thought to serve as situation-dependent affective signals and accomplish important communicative functions. In appetitive situations, rats produce 50 kHz USVs, whereas 22 kHz USVs occur in aversive situations. Reception of 50 kHz USVs induces social approach behavior, while 22 kHz USVs lead to freezing behavior. These opposite behavioral responses are paralleled by distinct brain activation patterns, with 50 kHz USVs, but not 22 kHz USVs, activating neurons in the nucleus accumbens (NAcc). The NAcc mediates appetitive behavior and is critically modulated by dopaminergic afferents that are known to encode the value of reward. Therefore, we hypothesized that 50 kHz USVs, but not 22 kHz USVs, elicit NAcc dopamine release. While recording dopamine signaling with fast-scan cyclic voltammetry, freely moving rats were exposed to playback of four acoustic stimuli via an ultrasonic speaker in random order: (1) 50 kHz USVs, (2) 22 kHz USVs, (3) time- and amplitude-matched white noise, and (4) background noise. Only presentation of 50 kHz USVs induced phasic dopamine release and elicited approach behavior toward the speaker. Both of these effects, neurochemical and behavioral, were most pronounced during initial playback, but then declined rapidly with subsequent presentations, indicating a close temporal relationship between the two measures. Moreover, the magnitudes of these effects during initial playback were significantly correlated. Collectively, our findings show that NAcc dopamine release encodes pro-social 50 kHz USVs, but not alarming 22 kHz USVs. Thus, our results support the hypothesis that these call types are processed in distinct neuroanatomical regions and establish a functional link between pro-social communicative signals and reward-related neurotransmission.
Keywords: dopamine; fast-scan cyclic voltammetry; nucleus accumbens; social behavior; ultrasonic communication.
Copyright © 2014 the authors 0270-6474/14/3410616-08$15.00/0.
Figures

Similar articles
-
Testing social acoustic memory in rats: effects of stimulus configuration and long-term memory on the induction of social approach behavior by appetitive 50-kHz ultrasonic vocalizations.Neurobiol Learn Mem. 2012 Sep;98(2):154-64. doi: 10.1016/j.nlm.2012.05.004. Epub 2012 Jun 4. Neurobiol Learn Mem. 2012. PMID: 22677211
-
22 kHz and 55 kHz ultrasonic vocalizations differentially influence neural and behavioral outcomes: Implications for modeling anxiety via auditory stimuli in the rat.Behav Brain Res. 2019 Mar 15;360:134-145. doi: 10.1016/j.bbr.2018.12.005. Epub 2018 Dec 3. Behav Brain Res. 2019. PMID: 30521931 Free PMC article.
-
Studying Socio-Affective Communication in Rats through Playback of Ultrasonic Vocalizations.Curr Protoc Neurosci. 2016 Apr 8;75:8.35.1-8.35.17. doi: 10.1002/cpns.7. Curr Protoc Neurosci. 2016. PMID: 27063787
-
Pro-social ultrasonic communication in rats: insights from playback studies.J Neurosci Methods. 2014 Aug 30;234:73-81. doi: 10.1016/j.jneumeth.2014.01.023. Epub 2014 Feb 4. J Neurosci Methods. 2014. PMID: 24508146 Review.
-
Rat 50-kHz ultrasonic vocalizations as a tool in studying neurochemical mechanisms that regulate positive emotional states.J Neurosci Methods. 2018 Dec 1;310:33-44. doi: 10.1016/j.jneumeth.2018.06.018. Epub 2018 Jun 26. J Neurosci Methods. 2018. PMID: 29959002 Review.
Cited by
-
Developmental differences in amygdala projection neuron activation associated with isolation-driven changes in social preference.Front Behav Neurosci. 2022 Aug 24;16:956102. doi: 10.3389/fnbeh.2022.956102. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36090658 Free PMC article.
-
Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error.Elife. 2022 Nov 11;11:e82711. doi: 10.7554/eLife.82711. Elife. 2022. PMID: 36366962 Free PMC article.
-
Hearing, touching, and multisensory integration during mate choice.Front Neural Circuits. 2022 Sep 20;16:943888. doi: 10.3389/fncir.2022.943888. eCollection 2022. Front Neural Circuits. 2022. PMID: 36247731 Free PMC article. Review.
-
Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns.Brain Sci. 2022 Oct 31;12(11):1474. doi: 10.3390/brainsci12111474. Brain Sci. 2022. PMID: 36358402 Free PMC article.
-
Rat behavior and dopamine release are modulated by conspecific distress.Elife. 2018 Nov 28;7:e38090. doi: 10.7554/eLife.38090. Elife. 2018. PMID: 30484770 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
