Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice
- PMID: 25100609
- PMCID: PMC4122806
- DOI: 10.1523/JNEUROSCI.0723-14.2014
Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice
Abstract
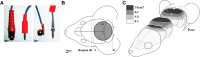
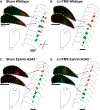
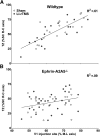
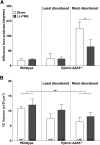
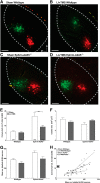
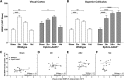
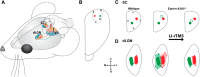
Repetitive transcranial magnetic stimulation (rTMS) is increasingly used as a treatment for neurological and psychiatric disorders. Although the induced field is focused on a target region during rTMS, adjacent areas also receive stimulation at a lower intensity and the contribution of this perifocal stimulation to network-wide effects is poorly defined. Here, we examined low-intensity rTMS (LI-rTMS)-induced changes on a model neural network using the visual systems of normal (C57Bl/6J wild-type, n = 22) and ephrin-A2A5(-/-) (n = 22) mice, the latter possessing visuotopic anomalies. Mice were treated with LI-rTMS or sham (handling control) daily for 14 d, then fluorojade and fluororuby were injected into visual cortex. The distribution of dorsal LGN (dLGN) neurons and corticotectal terminal zones (TZs) was mapped and disorder defined by comparing their actual location with that predicted by injection sites. In the afferent geniculocortical projection, LI-rTMS decreased the abnormally high dispersion of retrogradely labeled neurons in the dLGN of ephrin-A2A5(-/-) mice, indicating geniculocortical map refinement. In the corticotectal efferents, LI-rTMS improved topography of the most abnormal TZs in ephrin-A2A5(-/-) mice without altering topographically normal TZs. To investigate a possible molecular mechanism for LI-rTMS-induced structural plasticity, we measured brain derived neurotrophic factor (BDNF) in the visual cortex and superior colliculus after single and multiple stimulations. BDNF was upregulated after a single stimulation for all groups, but only sustained in the superior colliculus of ephrin-A2A5(-/-) mice. Our results show that LI-rTMS upregulates BDNF, promoting a plastic environment conducive to beneficial reorganization of abnormal cortical circuits, information that has important implications for clinical rTMS.
Keywords: LI-rTMS; corticotectal projection; critical period; ephrin-A2A5−/− mice; geniculocortical; plasticity.
Copyright © 2014 the authors 0270-6474/14/3410780-13$15.00/0.
Figures
Similar articles
-
Online LI-rTMS during a Visual Learning Task: Differential Impacts on Visual Circuit and Behavioral Plasticity in Adult Ephrin-A2A5-/- Mice.eNeuro. 2018 Feb 14;5(1):ENEURO.0163-17.2018. doi: 10.1523/ENEURO.0163-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464193 Free PMC article.
-
Manipulating the Level of Sensorimotor Stimulation during LI-rTMS Can Improve Visual Circuit Reorganisation in Adult Ephrin-A2A5-/- Mice.Int J Mol Sci. 2022 Feb 22;23(5):2418. doi: 10.3390/ijms23052418. Int J Mol Sci. 2022. PMID: 35269561 Free PMC article.
-
A role for ephrin-As in maintaining topographic organization in register across interconnected central visual pathways.Eur J Neurosci. 2010 Feb;31(4):613-22. doi: 10.1111/j.1460-9568.2010.07111.x. Eur J Neurosci. 2010. PMID: 20384808
-
What does low-intensity rTMS do to the cerebellum?Cerebellum. 2015 Feb;14(1):23-6. doi: 10.1007/s12311-014-0617-9. Cerebellum. 2015. PMID: 25346177 Review.
-
Repetitive Transcranial Magnetic Stimulation of the Brain After Ischemic Stroke: Mechanisms from Animal Models.Cell Mol Neurobiol. 2023 May;43(4):1487-1497. doi: 10.1007/s10571-022-01264-x. Epub 2022 Aug 2. Cell Mol Neurobiol. 2023. PMID: 35917043 Free PMC article. Review.
Cited by
-
Online LI-rTMS during a Visual Learning Task: Differential Impacts on Visual Circuit and Behavioral Plasticity in Adult Ephrin-A2A5-/- Mice.eNeuro. 2018 Feb 14;5(1):ENEURO.0163-17.2018. doi: 10.1523/ENEURO.0163-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464193 Free PMC article.
-
Low intensity repetitive transcranial magnetic stimulation modulates skilled motor learning in adult mice.Sci Rep. 2018 Mar 5;8(1):4016. doi: 10.1038/s41598-018-22385-8. Sci Rep. 2018. PMID: 29507375 Free PMC article.
-
Multiple blocks of intermittent and continuous theta-burst stimulation applied via transcranial magnetic stimulation differently affect sensory responses in rat barrel cortex.J Physiol. 2015 Feb 15;593(4):967-85. doi: 10.1113/jphysiol.2014.282467. Epub 2015 Jan 12. J Physiol. 2015. PMID: 25504571 Free PMC article.
-
Dorsal striatum c-Fos activity in perseverative ephrin-A2A5-/- mice and the cellular effect of low-intensity rTMS.Front Neural Circuits. 2023 Jun 15;17:1179096. doi: 10.3389/fncir.2023.1179096. eCollection 2023. Front Neural Circuits. 2023. PMID: 37396401 Free PMC article.
-
Interactions between Guidance Cues and Neuronal Activity: Therapeutic Insights from Mouse Models.Int J Mol Sci. 2023 Apr 9;24(8):6966. doi: 10.3390/ijms24086966. Int J Mol Sci. 2023. PMID: 37108129 Free PMC article. Review.
References
-
- Arias-Carrión O, Verdugo-Díaz L, Feria-Velasco A, Millán-Aldaco D, Gutiérrez AA, Hernández-Cruz A, Drucker-Colín R. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. 2004;78:16–28. doi: 10.1002/jnr.20235. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
