Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and peripheral factors in long-term tolerance
- PMID: 25100613
- PMCID: PMC4194165
- DOI: 10.1111/ajt.12816
Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and peripheral factors in long-term tolerance
Abstract
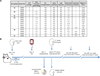
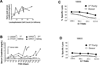
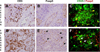
We have previously demonstrated that long-term tolerance (LTT) of an MHC class-I mismatched renal allograft can be achieved with a short course of cyclosporine. In order to examine regulatory mechanisms underlying tolerance in this model, we assessed the contributions of factors within the graft and in the peripheral blood for their relative roles in the maintenance of stable tolerance. Twelve LTT recipients of MHC class-I mismatched primary kidneys were subjected to a treatment consisting of donor-specific transfusion followed by leukapheresis, in order to remove peripheral leukocytes, including putative regulatory T cells (Tregs). Following treatment, 2 controls were followed clinically and 10 animals had the primary graft removed and received a second, donor-MHC-matched kidney. Neither control animal showed evidence of rejection, while 8 of 10 retransplanted animals developed either rejection crisis or full rejection of the second transplant. In vitro assays confirmed that the removed leukocytes were suppressive and that CD4(+) Foxp3(+) Treg reconstitution in blood and kidney grafts correlated with return to normal renal function in animals experiencing transient rejection crises. These data indicate that components of accepted kidney grafts as well as peripheral regulatory components both contribute to the tolerogenic environment required for tolerance of MHC class-I mismatched allotransplants.
Keywords: Animal models: porcine; basic (laboratory) research/science; immunosuppression/immune modulation; kidney transplantation/nephrology; tolerance: experimental; translational research/science.
© Copyright 2014 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures


References
-
- Fishbein JM, Rosengard BR, Gianello P, et al. Development of tolerance to class II mismatched renal transplants following a short course of cyclosporine therapy in miniature swine. Transplantation. 1994;57:1303–1308. - PubMed
-
- Fishbein JM, Gianello P, Nickeleit V, et al. Interleukin-2 reverses the ability of cyclosporine to induce tolerance to class I disparate kidney allografts in miniature swine. Transplant Proc. 1993;25:322–323. - PubMed
-
- Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

