FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice
- PMID: 25100706
- PMCID: PMC4435062
- DOI: 10.1095/biolreprod.114.118562
FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice
Abstract
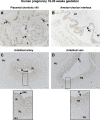
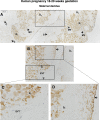
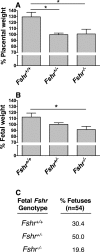
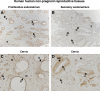
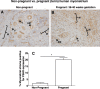
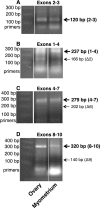
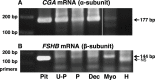
Expression and function of the follicle-stimulating hormone receptor (FSHR) in females were long thought to be limited to the ovary. Here, however, we identify extragonadal FSHR in both the human female reproductive tract and the placenta, and test its physiological relevance in mice. We show that in nonpregnant women FSHR is present on: endothelial cells of blood vessels in the endometrium, myometrium, and cervix; endometrial glands of the proliferative and secretory endometrium; cervical glands and the cervical stroma; and (at low levels) stromal cells and muscle fibers of the myometrium. In pregnant women, placental FSHR was detected as early as 8-10 wk of gestation and continued through term. It was expressed on: endothelial cells in fetal portions of the placenta and the umbilical cord; epithelial cells of the amnion; decidualized cells surrounding the maternal arteries in the maternal decidua; and the stromal cells and muscle fibers of the myometrium, with particularly strong expression at term. These findings suggest that FSHR expression is upregulated during decidualization and upregulated in myometrium as a function of pregnancy. The presence of FSHR in the placental vasculature suggests a role in placental angiogenesis. Analysis of genetically modified mice in which Fshr is lacking in fetal portions of the placenta revealed adverse effects on fetoplacental development. Our data further demonstrate FSHB and CGA mRNAs in placenta and uterus, consistent with potential local sources of FSH. Collectively, our data suggest heretofore unappreciated roles of extragonadal FSHR in female reproductive physiology.
Keywords: FSH receptor; cervix; follicle-stimulating hormone; gonadotropin; placenta; pregnancy; uterus.
© 2014 by the Society for the Study of Reproduction, Inc.
Figures





Similar articles
-
Follicle stimulating hormone receptor protein is expressed in ovine uterus during the estrous cycle and utero-placenta during early pregnancy: An immunohistochemical study.Acta Histochem. 2018 Jul;120(5):420-428. doi: 10.1016/j.acthis.2018.05.005. Epub 2018 May 11. Acta Histochem. 2018. PMID: 29754696
-
Deletion of fetoplacental Fshr inhibits fetal vessel angiogenesis in the mouse placenta.Mol Cell Endocrinol. 2018 Nov 15;476:79-83. doi: 10.1016/j.mce.2018.04.011. Epub 2018 Apr 30. Mol Cell Endocrinol. 2018. PMID: 29715497 Free PMC article.
-
Reduced expression of follicle stimulating hormone receptor mRNA and protein in pregnancies complicated by pre‑eclampsia.Mol Med Rep. 2017 Jul;16(1):367-372. doi: 10.3892/mmr.2017.6599. Epub 2017 May 18. Mol Med Rep. 2017. PMID: 28534997
-
FSH Actions and Pregnancy: Looking Beyond Ovarian FSH Receptors.Endocrinology. 2018 Dec 1;159(12):4033-4042. doi: 10.1210/en.2018-00497. Endocrinology. 2018. PMID: 30395176 Free PMC article. Review.
-
Endogenous, tissue-resident stem/progenitor cells in gonads and bone marrow express FSHR and respond to FSH via FSHR-3.J Ovarian Res. 2021 Oct 30;14(1):145. doi: 10.1186/s13048-021-00883-0. J Ovarian Res. 2021. PMID: 34717703 Free PMC article. Review.
Cited by
-
Follicle-Stimulating Hormone Promotes the Development of Endometrial Cancer In Vitro and In Vivo.Int J Environ Res Public Health. 2022 Nov 20;19(22):15344. doi: 10.3390/ijerph192215344. Int J Environ Res Public Health. 2022. PMID: 36430063 Free PMC article.
-
Trajectories of Blood Pressure in Midlife Women: Does Menopause Matter?Circ Res. 2022 Feb 4;130(3):312-322. doi: 10.1161/CIRCRESAHA.121.319424. Epub 2022 Jan 6. Circ Res. 2022. PMID: 35113663 Free PMC article.
-
Genetic Polymorphisms in miR-604A>G, miR-938G>A, miR-1302-3C>T and the Risk of Idiopathic Recurrent Pregnancy Loss.Int J Mol Sci. 2021 Jun 7;22(11):6127. doi: 10.3390/ijms22116127. Int J Mol Sci. 2021. PMID: 34200157 Free PMC article.
-
Extragonadal Actions of FSH: A Critical Need for Novel Genetic Models.Endocrinology. 2018 Jan 1;159(1):2-8. doi: 10.1210/en.2017-03118. Endocrinology. 2018. PMID: 29236987 Free PMC article. Review.
-
Effect of human amniotic epithelial cells on ovarian function, fertility and ovarian reserve in primary ovarian insufficiency rats and analysis of underlying mechanisms by mRNA sequencing.Am J Transl Res. 2020 Jul 15;12(7):3234-3254. eCollection 2020. Am J Transl Res. 2020. PMID: 32774697 Free PMC article.
References
-
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. - PubMed
-
- Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, Fromont G, Hai MT, Ghinea N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630. - PubMed
-
- Mizrachi D, Shemesh M. Follicle-stimulating hormone receptor and its messenger ribonucleic acid are present in the bovine cervix and can regulate cervical prostanoid synthesis. Biol Reprod. 1999;61:776–784. - PubMed
-
- Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

