Distinguishing closely related amyloid precursors using an RNA aptamer
- PMID: 25100729
- PMCID: PMC4175327
- DOI: 10.1074/jbc.M114.595066
Distinguishing closely related amyloid precursors using an RNA aptamer
Abstract
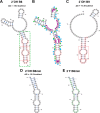
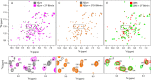
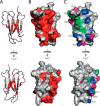
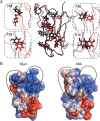
Although amyloid fibrils assembled in vitro commonly involve a single protein, fibrils formed in vivo can contain multiple protein sequences. The amyloidogenic protein human β2-microglobulin (hβ2m) can co-polymerize with its N-terminally truncated variant (ΔN6) in vitro to form hetero-polymeric fibrils that differ from their homo-polymeric counterparts. Discrimination between the different assembly precursors, for example by binding of a biomolecule to one species in a mixture of conformers, offers an opportunity to alter the course of co-assembly and the properties of the fibrils formed. Here, using hβ2m and its amyloidogenic counterpart, ΔΝ6, we describe selection of a 2'F-modified RNA aptamer able to distinguish between these very similar proteins. SELEX with a N30 RNA pool yielded an aptamer (B6) that binds hβ2m with an EC50 of ∼200 nM. NMR spectroscopy was used to assign the (1)H-(15)N HSQC spectrum of the B6-hβ2m complex, revealing that the aptamer binds to the face of hβ2m containing the A, B, E, and D β-strands. In contrast, binding of B6 to ΔN6 is weak and less specific. Kinetic analysis of the effect of B6 on co-polymerization of hβ2m and ΔN6 revealed that the aptamer alters the kinetics of co-polymerization of the two proteins. The results reveal the potential of RNA aptamers as tools for elucidating the mechanisms of co-assembly in amyloid formation and as reagents able to discriminate between very similar protein conformers with different amyloid propensity.
Keywords: Amyloid; Amyloid Fibril; Amyloid Precursor; Aptamer; Co-polymerization; Protein Aggregation; Protein Folding; RNA Aptamer; Structural Biology; β2-Microglobulin.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

