Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis
- PMID: 25100740
- PMCID: PMC4176763
- DOI: 10.1126/scitranslmed.3008930
Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis
Abstract
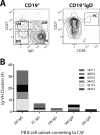
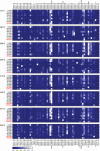
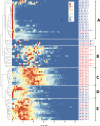
In multiple sclerosis (MS), lymphocyte--in particular B cell--transit between the central nervous system (CNS) and periphery may contribute to the maintenance of active disease. Clonally related B cells exist in the cerebrospinal fluid (CSF) and peripheral blood (PB) of MS patients; however, it remains unclear which subpopulations of the highly diverse peripheral B cell compartment share antigen specificity with intrathecal B cell repertoires and whether their antigen stimulation occurs on both sides of the blood-brain barrier. To address these questions, we combined flow cytometric sorting of PB B cell subsets with deep immune repertoire sequencing of CSF and PB B cells. Immunoglobulin (IgM and IgG) heavy chain variable (VH) region repertoires of five PB B cell subsets from MS patients were compared with their CSF Ig-VH transcriptomes. In six of eight patients, we identified peripheral CD27(+)IgD(-) memory B cells, CD27(hi)CD38(hi) plasma cells/plasmablasts, or CD27(-)IgD(-) B cells that had an immune connection to the CNS compartment. Pinpointing Ig class-switched B cells as key component of the immune axis thought to contribute to ongoing MS disease activity strengthens the rationale of current B cell-targeting therapeutic strategies and may lead to more targeted approaches.
Copyright © 2014, American Association for the Advancement of Science.
Figures
Comment in
-
Street-experienced peripheral B cells traffic to the brain.Sci Transl Med. 2014 Aug 6;6(248):248fs31. doi: 10.1126/scitranslmed.3009919. Sci Transl Med. 2014. PMID: 25100737 Free PMC article.
References
-
- Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Annals of neurology. 2008;63:395–400. - PubMed
-
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, Group HT. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. - PubMed
-
- Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, Yin M, Leppert D, Glanzman R, Tinbergen J, Hauser SL. Ocrelizumab in relapsingremitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. - PubMed
-
- Obermeier B, Lovato L, Mentele R, Bruck W, Forne I, Imhof A, Lottspeich F, Turk KW, Willis SN, Wekerle H, Hohlfeld R, Hafler DA, O'Connor KC, Dornmair K. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. Journal of neuroimmunology. 2011;233:245–248. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

