Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice
- PMID: 25100745
- PMCID: PMC4287930
- DOI: 10.15252/emmm.201404228
Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice
Abstract
Insulin-like growth factor 2 (IGF2) was recently found to play a critical role in memory consolidation in rats and mice, and hippocampal or systemic administration of recombinant IGF2 enhances memory. Here, using a gene therapy-based approach with adeno-associated virus (AAV), we show that IGF2 overexpression in the hippocampus of aged wild-type mice enhances memory and promotes dendritic spine formation. Furthermore, we report that IGF2 expression decreases in the hippocampus of patients with Alzheimer's disease, and this leads us to hypothesize that increased IGF2 levels may be beneficial for treating the disease. Thus, we used the AAV system to deliver IGF2 or IGF1 into the hippocampus of the APP mouse model Tg2576 and demonstrate that IGF2 and insulin-like growth factor 1 (IGF1) rescue behavioural deficits, promote dendritic spine formation and restore normal hippocampal excitatory synaptic transmission. The brains of Tg2576 mice that overexpress IGF2 but not IGF1 also show a significant reduction in amyloid levels. This reduction probably occurs through an interaction with the IGF2 receptor (IGF2R). Hence, IGF2 and, to a lesser extent, IGF1 may be effective treatments for Alzheimer's disease.
Keywords: Alzheimer's disease; IGF1; IGF2; IGF2R; synaptic plasticity.
© 2014 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
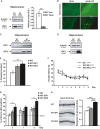
Quantitative Western blot analysis of hippocampal extracts that reveal a decrease in IGF2 in the hippocampus of aged (15-month-old) WT mice compared to 7-month-old WT animals. Data are expressed as arbitrary units (mean ± SEM) with respect to the WT 7-month-old mice (unpaired two-tailed Student'st-test,n = 4, **P = 0.01).
Two months after the intrahippocampal administration of AAV8-GFP, GFP was detected in the pyramidal cells of CA1 and in neurons of the entorhinal cortex (Cx). No signal was detected in sham-injected animals. Scale bar = 10 μm.
Gel analysis of PCR products obtained with the primers designed to determine the presence of AAV-mediated expression of IGF1 or IGF2 (WT AAV-IGF) in the hippocampus of WT mice. No band was detected in the sham-injected mice (WT sham).
Western blot analysis of hippocampal extracts that reveal the overexpression of the IGF2 protein in the AAV-IGF2-injected mice compared to the sham-injected animals.
Contextual freezing responses of aged WT mice 2 months after AAV-IGF2 injection (WT IGF2) showed a significant enhancement of memory retention measured as % freezing compared to the sham-injected mice (WT). In this, and all subsequent figures, results are expressed as mean ± SEM (Kruskal–Wallis followed by Mann–WhitneyU-test,n = 10–12, **P = 0.0074).
Escape latency to the hidden platform in the Morris water maze test for aged WT, WT IGF1 and WT IGF2 mice. Latency to reach the platform decreased in every group as the training sessions progressed (non-parametric Friedman test,n = 10–12, **P = 0.0029 WT, *P = 0.05 WT IGF1, ***P = 0.00026 WT IGF2). No differences were observed among the groups.
Percentage of time spent searching for the target quadrant in the probe test (on days 4, 7 and 9). On days 4 and 9, WT IGF2 mice showed significant improvement in memory retention, measured as % of time spent in the right quadrant, compared to WT mice (one-way ANOVA followed by Scheffe'spost hoc test,n = 10–12, *P = 0.043 day 4, *P = 0.050 day 9).
Representative images of Golgi-impregnated apical dendrites of CA1 hippocampal pyramidal neurons. Scale bar = 10 μm (left panel). Quantification of overall spine density in 20-month-old WT mice (right panel). Spine density significantly increased in WT IGF1 and WT IGF2 mice compared to sham WT mice (Kruskal–Wallis followed by Mann–WhitneyU-test,n = 36 neurons, *P = 0.025 WT versus WT IGF1, ***P = 1.03E-11 WT versus WT IGF2. The increase was greater for WT IGF2 mice, where spine density significantly increased compared to WT IGF1 (***P = 4.70E-08).

Hippocampal (n = 8) and entorhinal cortex (Cx,n = 6) from patients with AD compared to corresponding non-demented controls (n = 5 andn = 6 respectively). In this, and all subsequent figures, data are expressed as arbitrary units (mean ± SEM) with respect to their respective controls (unpaired two-tailed Student'st-test,n = 5–8, **P = 0.007).
Seven-month-old Tg2576 mice hippocampus compared to age-matched WT mice (unpaired two-tailed Student'st-test,n = 4, **P = 0.010).
Neuronal cultures of Tg2576 mice and neuronal cultures of WT mice exposed to conditioned medium (CM) obtained from Tg2576 primary neurons for 24 h compared to their respective control (one-way ANOVA followed by Scheffe'spost hoc test,n = 5–6, ***P = 0.0006 WT versus Tg2576, ***P = 0.0003 WT versus WT + CM).
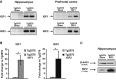
Gel analysis of PCR products to determine the presence of AAV-mediated IGF1 and IGF2 expression. AAV-IGF-injected Tg2576 mice showed a PCR product corresponding to IGF expression in the hippocampus and the prefrontal cortex. No band was detected in the sham-injected mice (Tg2576 sham).
Quantification of the relative expression of IGF transcripts by real-time PCR analysis using primers designed against the corresponding cDNAs of the murine IGFs. AAV-IGF-injected Tg2576 mice showed a robust increase in the corresponding IGF compared to their respective controls (Tg2576 sham). Data are expressed as the fold change (mean ± SEM) with respect to the controls (unpaired two-tailed Student'st-test,n = 5–6, *P = 0.036 Tg2576 IGF1, *P = 0.050 Tg2576 IGF2).
Western blot analysis of hippocampal extracts revealing the overexpression of IGF2 protein in the AAV-IGF2-injected mice (Tg2576 IGF2) compared to Tg2576 sham.
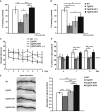
Contextual freezing responses of 16-month-old Tg2576 mice were impaired when compared to controls (WT), and significantly improved 4 months after the AAV-IGF1 (Tg2576 IGF1) and AAV-IGF2 (Tg2576 IGF2) injections. In this, and all subsequent figures, the results are expressed as mean ± SEM (Kruskal–Wallis followed by Mann–WhitneyU-test,n = 10–12, **P = 0.008 WT versus Tg2576, *P = 0.041 Tg2576 versus Tg2576 IGF1, ***P = 0.0002 Tg2576 versus Tg2576 IGF2, **P = 0.0029 Tg2576 IGF1 versus Tg2576 IGF2).
Memory impairments in Tg2576 mice progressed with age, and at 20 months, only Tg2576 IGF2 mice showed a significant improvement in memory retention, measured as % contextual freezing, compared to sham-injected Tg2576 mice (Tg2576) (Kruskal–Wallis followed by Mann–WhitneyU-test,n = 10–12, **P = 0.0014 WT versus Tg2576, *P = 0.037 WT versus Tg2576 IGF1, *P = 0.039 WT versus Tg2576 IGF2, *P = 0.050 Tg2576 versus Tg2576 IGF2).
Escape latency to the hidden platform in the Morris water maze test for WT, Tg2576, Tg2576 IGF1 and Tg2576 IGF2 mice. Latency to reach the platform decreased in WT and Tg2576 IGF2 mice as the training sessions progressed. No such change was observed in Tg2576 IGF1 or Tg2576 mice (non-parametric Friedman test,n = 10–12, **P = 0.002 WT, **P = 0.005 Tg2576 IGF2).
Percentage of time spent searching for the target quadrant in the probe test (days 4, 7 and 9). Tg2576 mice performed significantly more poorly than WT mice. AAV-IGF2-injected groups performed similarly to WT mice on days 7 and 9 (one-way ANOVA followed by Scheffe'spost hoc test,n = 10–12, *P = 0.045 WT versus Tg2576 day 7, *P = 0.05 WT versus Tg2576 IGF1 day 7, *P = 0.049 Tg2576 versus Tg2576 IGF2 day 7, **P = 0.007 WT versus Tg2576 day 9, *P = 0.044 WT versus Tg2576 IGF1 day 9, *P = 0.035 Tg2576 versus Tg2576 IGF2 day 9).
Representative images of Golgi-impregnated apical dendrites of CA1 hippocampal pyramidal neurons. Scale bar = 10 μm (left panel). Quantification of overall spine density in 20-month-old Tg2576 mice (right panel). Significant reduction in spine density was detected in 20-month-old Tg2576 mice compared to WT mice (Kruskal–Wallis followed by Mann–WhitneyU-test,n = 27–36 neurons, ***P = 0.0009 WT versus Tg2576). AAV-IGF1 treatment in Tg2576 mice (Tg2576 IGF1) completely reversed spine loss, which returned to littermate control levels (WT) (***P = 1.67E-05 Tg2576 versus Tg2576 IGF1). AAV-IGF2 treatment (Tg2576 IGF2) increased spine density to above control values (***P = 6.05E-08 Tg2576 IGF2 versus WT, ***P = 1.94E-12 Tg2576 IGF2 versus Tg2576, ***P = 2.86E-06 Tg2576 IGF2 versus Tg2576 IGF1).

Representative traces of spontaneous AMPA EPSCs were isolated from the first 15 min after whole cell configuration.
Cumulative probability analysis of the distribution of inter-event intervals between mEPSCs showed a very significant difference between WT and Tg2576 (non-parametric Kolmogorov–Smirnov test,n = 10,P = 0.0002). Treatment with IGF1 and IGF2 rescued the frequency of mEPSCs to values that were no different from those of the control animals (P = 0.0129 Tg2576 versus Tg2576 IGF1,P < 0.0001 Tg2576 versus Tg2576 IGF2).
Average mEPSC interval between the first 100 events of 10 cells per treatment (one-way ANOVA followed by Tukey'spost hoc test,n = 10, ***P < 0.001).
Cumulative probability analysis of mEPSC amplitude showed a difference between Tg2576 and WT (non-parametric Kolmogorov–Smirnov test,n = 10,P = 0.0003). The mEPSC amplitude of the treated groups was not rescued to the values of the WT animals (P = 0.3136 Tg2576 versus Tg2576 IGF1,P = 0.4658 Tg2576 versus Tg2576 IGF2).
Average amplitude of the first 100 events of 10 cells per treatment (one-way ANOVA followed by Tukey'spost hoc test,n = 10, ***P < 0.001).

Representative hippocampal sections of sham- (Tg2576), AAV-IGF1- (Tg2576 IGF1) and AAV-IGF2- (Tg2576 IGF2) treated Tg2576 mice are shown (left panel). Scale bar = 100 μm. Amyloid burden quantification (right panel). Multiple extracellular deposits stained with 6E10 antiserum were detected in Tg2576 and Tg2576 IGF1 mice. Amyloid burden is reduced in the hippocampus of Tg2576 IGF2 mice (n = 3–5); however, no significant differences were found.
Aβ42 and Aβ40 concentration determined by ELISA in the prefrontal and parietotemporal cortices (Cx) of Tg2576 mice and Tg2576 IGF1 mice showed similar values. Interestingly, Tg2576 IGF2 mice exhibited a significant reduction in Aβ42 (one-way ANOVA followed by Scheffe'spost hoc test,n = 7–8, *P = 0.050 Tg2576 versus Tg2576 IGF2 prefrontal Cx, **P = 0.007 Tg2576 versus Tg2576 IGF2 parietotemporal Cx, *P = 0.038 Tg2576 IGF1 versus Tg2576 IGF2 parietotemporal Cx) and Aβ40 (*P = 0.019 Tg2576 versus Tg2576 IGF2 prefrontal Cx, *P = 0.014 Tg2576 IGF1 versus Tg2576 IGF2 prefrontal Cx, **P = 0.003 Tg2576 versus Tg2576 IGF2 parietotemporal Cx, *P = 0.038 Tg2576 IGF1 versus Tg2576 IGF2 parietotemporal Cx) cortical levels. Data are the mean ± SEM.
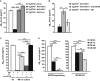
ELISA analysis to detect Aβ42 levels in the media of Tg2576 primary neuronal culture infected with AAV-IGF1, AAV-IGF2 or AAV-Luc. A significant depletion in extracellular Aβ42 levels was detected in the media of AAV-IGF2-infected neurons. In this, and all subsequent figures, data (mean ± SEM) are expressed in pg of Aβ42/ml of conditioned medium. Bars represent the analysis from three independent experiments (one-way ANOVA followed by Scheffe'spost hoc test,n = 3–4, ***P = 3.12E-08 Tg2576 control versus Tg2576 + AAV-IGF2, ***P = 1.91E-08 Tg2576 + AAV-IGF2 versus Tg2576 + AAV-IGF1, ***P = 2.39E-09 Tg2576 + AAV-IGF2 versus Tg2576 + AAV-Luc).
ELISA analysis to detect Aβ42 levels in the medium from Tg2576 primary neuronal cultures transfected with AAV-IGF2. Pre-incubation with anti-IGF2R antibody partially prevented Aβ42 clearance in the media of infected neurons. Bars represent the analysis from three independent experiments (one-way ANOVA followed by Scheffe'spost hoc test,n = 3–4, **P = 0.009, *P = 0.036).
ELISA analysis to detect Aβ42 levels in media obtained from WT primary neuronal cultures exposed to conditioned medium (CM) from Tg2576 neurons for 48 h. Full depletion in extracellular Aβ42 occurred after 48 h; this was prevented by pre-incubation with proteinase inhibitors (PI) and/or anti-IGF2R antibody (one-way ANOVA followed by Scheffe'spost hoc test,n = 3–4, ***P = 2.65E-09 CM versus CM + PI, ***P = 3.56E-05 CM versus CM + anti-IGF2R, ***P = 1.26E-11 CM versus CM + PI + anti-IGF2R, ***P = 1.57E-08 CM + PI versus CM + PI + anti-IGF2R, ***P = 7.80E-11 CM + anti-IGF2R versus CM + PI + anti-IGF2R).
Analysis by sandwich ELISA of Aβ42 levels at different time intervals (0, 1, 4 and 24 h) in the media of hepatic cell lines (BNL.CL.2 and HepA129; IGF2R-expressing and IGF2-KO, respectively) exposed to conditioned medium from Tg2576 primary neurons (CM). After 4 h of incubation, extracellular Aβ42 was completely eliminated from the IGF2R-expressing cell medium (one-way ANOVA followed by Scheffe'spost hoc test,n = 3–4, **P = 0.002 CM 0 h versus CM 1 h, ***P = 1.99E-05 CM 0 h versus CM 4 h, ***P = 8.23E-06 CM 0 h versus CM 24 h, **P = 0.006 CM 1 h versus CM 4 h, **P = 0.006 CM 1 h versus CM 24 h, *P = 0.02 CM 4 h versus CM 24 h), but not from the IGF2R-KO cell medium, where Aβ42 was detected even after 24 h (*P = 0.011 CM 0 h versus CM 1 h, **P = 0.009 CM 0 h versus CM 4 h, ***P = 0.0001 CM 0 h versus CM 24 h, **P = 0.002 CM 1 h versus CM 24 h, **P = 0.0014 CM 4 h versus CM 24 h). Bars represent the analysis from three independent measurements.
References
-
- Braak H, Braak E, Yilmazer D, Bohl J. Functional anatomy of human hippocampal formation and related structures. J Child Neurol. 1996;11:265–275. - PubMed
-
- Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J, Clemenson GD, Jr, Suh H, Couillard-Despres S, Aigner L, et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci. 2012;32:3376–3387. - PMC - PubMed
-
- Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1999;31:242–246. - PubMed
-
- Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther. 2005;16:781–791. - PubMed
-
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

