Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens
- PMID: 25100818
- PMCID: PMC4187753
- DOI: 10.1128/JCM.01637-14
Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens
Abstract
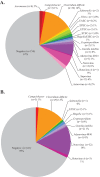
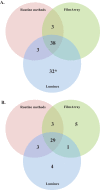
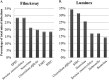
The detection of pathogens associated with gastrointestinal disease may be important in certain patient populations, such as immunocompromised hosts, the critically ill, or individuals with prolonged disease that is refractory to treatment. In this study, we evaluated two commercially available multiplex panels (the FilmArray gastrointestinal [GI] panel [BioFire Diagnostics, Salt Lake City, UT] and the Luminex xTag gastrointestinal pathogen panel [GPP] [Luminex Corporation, Toronto, Canada]) using Cary-Blair stool samples (n = 500) submitted to our laboratory for routine GI testing (e.g., culture, antigen testing, microscopy, and individual real-time PCR). At the time of this study, the prototype (non-FDA-cleared) FilmArray GI panel targeted 23 pathogens (14 bacterial, 5 viral, and 4 parasitic), and testing of 200 μl of Cary-Blair stool was recommended. In contrast, the Luminex GPP assay was FDA cleared for the detection of 11 pathogens (7 bacterial, 2 viral, and 2 parasitic), but had the capacity to identify 4 additional pathogens using a research-use-only protocol. Importantly, the Luminex assay was FDA cleared for 100 μl raw stool; however, 100 μl Cary-Blair stool was tested by the Luminex assay in this study. Among 230 prospectively collected samples, routine testing was positive for one or more GI pathogens in 19 (8.3%) samples, compared to 76 (33.0%) by the FilmArray and 69 (30.3%) by the Luminex assay. Clostridium difficile (12.6 to 13.9% prevalence) and norovirus genogroup I (GI)/GII (5.7 to 13.9% prevalence) were two of the pathogens most commonly detected by both assays among prospective samples. Sapovirus was also commonly detected (5.7% positive rate) by the FilmArray assay. Among 270 additional previously characterized samples, both multiplex panels demonstrated high sensitivity (>90%) for the majority of targets, with the exception of several pathogens, notably Aeromonas sp. (23.8%) by FilmArray and Yersinia enterocolitica (48.1%) by the Luminex assay. Interestingly, the FilmArray and Luminex panels identified mixed infections in 21.1% and 13.0% of positive prospective samples, respectively, compared to only 8.3% by routine methods.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

