The 3' untranslated region of Pea Enation Mosaic Virus contains two T-shaped, ribosome-binding, cap-independent translation enhancers
- PMID: 25100834
- PMCID: PMC4178710
- DOI: 10.1128/JVI.01433-14
The 3' untranslated region of Pea Enation Mosaic Virus contains two T-shaped, ribosome-binding, cap-independent translation enhancers
Abstract
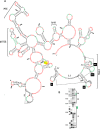
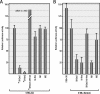
Many plant viruses without 5' caps or 3' poly(A) tails contain 3' proximal, cap-independent translation enhancers (3'CITEs) that bind to ribosomal subunits or translation factors thought to assist in ribosome recruitment. Most 3'CITEs participate in a long-distance kissing-loop interaction with a 5' proximal hairpin to deliver ribosomal subunits to the 5' end for translation initiation. Pea Enation Mosaic Virus (PEMV) contains two adjacent 3'CITEs in the center of its 703-nucleotide 3' untranslated region (3'UTR), the ribosome-binding, kissing-loop T-shaped structure (kl-TSS) and eukaryotic translation initiation factor 4E-binding Panicum mosaic virus-like translation enhance (PTE). We now report that PEMV contains a third, independent 3'CITE located near the 3' terminus. This 3'CITE is composed of three hairpins and two pseudoknots, similar to the TSS 3'CITE of the carmovirus Turnip crinkle virus (TCV). As with the TCV TSS, the PEMV 3'TSS is predicted to fold into a T-shaped structure that binds to 80S ribosomes and 60S ribosomal subunits. A small hairpin (kl-H) upstream of the 3'TSS contains an apical loop capable of forming a kissing-loop interaction with a 5' proximal hairpin and is critical for the accumulation of full-length PEMV in protoplasts. Although the kl-H and 3'TSS are dispensable for the translation of a reporter construct containing the complete PEMV 3'UTR in vitro, deleting the normally required kl-TSS and PTE 3'CITEs and placing the kl-H and 3'TSS proximal to the reporter termination codon restores translation to near wild-type levels. This suggests that PEMV requires three 3'CITEs for proper translation and that additional translation enhancers may have been missed if reporter constructs were used in 3'CITE identification. Importance: The rapid life cycle of viruses requires efficient translation of viral-encoded proteins. Many plant RNA viruses contain 3' cap-independent translation enhancers (3'CITEs) to effectively compete with ongoing host translation. Since only single 3'CITEs have been identified for the vast majority of individual viruses, it is widely accepted that this is sufficient for a virus's translational needs. Pea Enation Mosaic Virus possesses a ribosome-binding 3'CITE that can connect to the 5' end through an RNA-RNA interaction and an adjacent eukaryotic translation initiation factor 4E-binding 3'CITE. We report the identification of a third 3'CITE that binds weakly to ribosomes and requires an upstream hairpin to form a bridge between the 3' and 5' ends. Although both ribosome-binding 3'CITEs are critical for virus accumulation in vivo, only the CITE closest to the termination codon of a reporter open reading frame is active, suggesting that artificial constructs used for 3'CITE identification may underestimate the number of CITEs that participate in translation.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures








Similar articles
-
Identification of Novel 5' and 3' Translation Enhancers in Umbravirus-Like Coat Protein-Deficient RNA Replicons.J Virol. 2022 Apr 13;96(7):e0173621. doi: 10.1128/jvi.01736-21. Epub 2022 Mar 17. J Virol. 2022. PMID: 35297668 Free PMC article.
-
Conserved Structure Associated with Different 3'CITEs Is Important for Translation of Umbraviruses.Viruses. 2023 Feb 27;15(3):638. doi: 10.3390/v15030638. Viruses. 2023. PMID: 36992347 Free PMC article.
-
The kissing-loop T-shaped structure translational enhancer of Pea enation mosaic virus can bind simultaneously to ribosomes and a 5' proximal hairpin.J Virol. 2013 Nov;87(22):11987-2002. doi: 10.1128/JVI.02005-13. Epub 2013 Aug 28. J Virol. 2013. PMID: 23986599 Free PMC article.
-
3'UTRs of carmoviruses.Virus Res. 2015 Aug 3;206:27-36. doi: 10.1016/j.virusres.2015.01.023. Epub 2015 Feb 4. Virus Res. 2015. PMID: 25662021 Review.
-
3' Cap-independent translation enhancers of positive-strand RNA plant viruses.Curr Opin Virol. 2011 Nov;1(5):373-80. doi: 10.1016/j.coviro.2011.10.002. Epub 2011 Oct 24. Curr Opin Virol. 2011. PMID: 22440838 Review.
Cited by
-
Panicum Mosaic Virus and Its Satellites Acquire RNA Modifications Associated with Host-Mediated Antiviral Degradation.mBio. 2019 Aug 27;10(4):e01900-19. doi: 10.1128/mBio.01900-19. mBio. 2019. PMID: 31455653 Free PMC article.
-
Translational Control in Virus-Infected Cells.Cold Spring Harb Perspect Biol. 2019 Mar 1;11(3):a033001. doi: 10.1101/cshperspect.a033001. Cold Spring Harb Perspect Biol. 2019. PMID: 29891561 Free PMC article. Review.
-
Different RNA Elements Control Viral Protein Synthesis in Polerovirus Isolates Evolved in Separate Geographical Regions.Int J Mol Sci. 2022 Oct 19;23(20):12503. doi: 10.3390/ijms232012503. Int J Mol Sci. 2022. PMID: 36293360 Free PMC article.
-
The 3' Untranslated Region of a Plant Viral RNA Directs Efficient Cap-Independent Translation in Plant and Mammalian Systems.Pathogens. 2019 Feb 28;8(1):28. doi: 10.3390/pathogens8010028. Pathogens. 2019. PMID: 30823456 Free PMC article.
-
Complete Nucleotide Sequence, Genome Organization, and Comparative Genomic Analyses of Citrus Yellow-Vein Associated Virus (CYVaV).Front Microbiol. 2021 Jun 8;12:683130. doi: 10.3389/fmicb.2021.683130. eCollection 2021. Front Microbiol. 2021. PMID: 34168635 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

