trans-Protease activity and structural insights into the active form of the alphavirus capsid protease
- PMID: 25100849
- PMCID: PMC4248945
- DOI: 10.1128/JVI.01692-14
trans-Protease activity and structural insights into the active form of the alphavirus capsid protease
Abstract
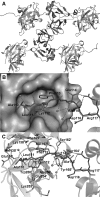
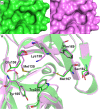
The alphavirus capsid protein (CP) is a serine protease that possesses cis-proteolytic activity essential for its release from the nascent structural polyprotein. The released CP further participates in viral genome encapsidation and nucleocapsid core formation, followed by its attachment to glycoproteins and virus budding. Thus, protease activity of the alphavirus capsid is a potential antialphaviral target to arrest capsid release, maturation, and structural polyprotein processing. However, the discovery of capsid protease inhibitors has been hampered due to the lack of a suitable screening assay and of the crystal structure in its active form. Here, we report the development of a trans-proteolytic activity assay for Aura virus capsid protease (AVCP) based on fluorescence resonance energy transfer (FRET) for screening protease inhibitors. Kinetic parameters using fluorogenic peptide substrates were estimated, and the K(m) value was found to be 2.63 ± 0.62 μM while the k(cat)/K(m) value was 4.97 × 10(4) M(-1) min(-1). Also, the crystal structure of the trans-active form of AVCP has been determined to 1.81-Å resolution. Structural comparisons of the active form with the crystal structures of available substrate-bound mutant and inactive blocked forms of the capsid protease identify conformational changes in the active site, the oxyanion hole, and the substrate specificity pocket residues, which could be critical for rational drug design. IMPORTANCE The alphavirus capsid protease is an attractive antiviral therapeutic target. In this study, we have described the formerly unappreciated trans-proteolytic activity of the enzyme and for the first time have developed a FRET-based protease assay for screening capsid protease inhibitors. Our structural studies unveil the structural features of the trans-active protease, which has been previously proposed to exist in the natively unfolded form (M. Morillas, H. Eberl, F. H. Allain, R. Glockshuber, and E. Kuennemann, J. Mol. Biol. 376:721-735, 2008, doi:http://dx.doi.org/10.1016/j.jmb.2007.11.055). The different enzymatic forms have been structurally compared to reveal conformational variations in the active and substrate binding sites. The flexible active-site residue Ser218, the disordered C-terminal residues after His261, and the presence of a water molecule in the oxyanion hole of AVCPΔ2 (AVCP with a deletion of the last two residues at the C terminus) reveal the effect of the C-terminal Trp267 deletion on enzyme structure. New structural data reported in this study along with the fluorogenic assay will be useful in substrate specificity characterization, high-throughput protease inhibitor screening, and structure-based development of antiviral drugs.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

