Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease
- PMID: 25101001
- PMCID: PMC4102118
- DOI: 10.3389/fphys.2014.00260
Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease
Abstract
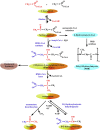
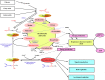
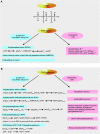
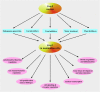
We provide a comprehensive review of the role of β-hydroxybutyrate (β-OHB), its linear polymer poly-β-hydroxybutyrate (PHB), and inorganic polyphosphate (polyP) in mammalian health and disease. β-OHB is a metabolic intermediate that constitutes 70% of ketone bodies produced during ketosis. Although ketosis has been generally considered as an unfavorable pathological state (e.g., diabetic ketoacidosis in type-1 diabetes mellitus), it has been suggested that induction of mild hyperketonemia may have certain therapeutic benefits. β-OHB is synthesized in the liver from acetyl-CoA by β-OHB dehydrogenase and can be used as alternative energy source. Elevated levels of PHB are associated with pathological states. In humans, short-chain, complexed PHB (cPHB) is found in a wide variety of tissues and in atherosclerotic plaques. Plasma cPHB concentrations correlate strongly with atherogenic lipid profiles, and PHB tissue levels are elevated in type-1 diabetic animals. However, little is known about mechanisms of PHB action especially in the heart. In contrast to β-OHB, PHB is a water-insoluble, amphiphilic polymer that has high intrinsic viscosity and salt-solvating properties. cPHB can form non-specific ion channels in planar lipid bilayers and liposomes. PHB can form complexes with polyP and Ca(2+) which increases membrane permeability. The biological roles played by polyP, a ubiquitous phosphate polymer with ATP-like bonds, have been most extensively studied in prokaryotes, however polyP has recently been linked to a variety of functions in mammalian cells, including blood coagulation, regulation of enzyme activity in cancer cells, cell proliferation, apoptosis and mitochondrial ion transport and energy metabolism. Recent evidence suggests that polyP is a potent activator of the mitochondrial permeability transition pore in cardiomyocytes and may represent a hitherto unrecognized key structural and functional component of the mitochondrial membrane system.
Keywords: cardiovascular disease; heart failure; inorganic polyphosphate; mitochondrial permeability transition pore; poly-β-hydroxybutyrate; β-hydroxybutyrate.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

