Time Course of Corticospinal Excitability and Autonomic Function Interplay during and Following Monopolar tDCS
- PMID: 25101009
- PMCID: PMC4104833
- DOI: 10.3389/fpsyt.2014.00086
Time Course of Corticospinal Excitability and Autonomic Function Interplay during and Following Monopolar tDCS
Abstract
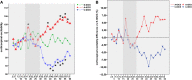
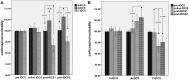
While polarity-specific after-effects of monopolar transcranial direct current stimulation (tDCS) on corticospinal excitability are well-documented, modulation of vital parameters due to current spread through the brainstem is still a matter of debate, raising potential concerns about its use through the general public, as well as for neurorehabilitation purposes. We monitored online and after-effects of monopolar tDCS (primary motor cortex) in 10 healthy subjects by adopting a neuronavigated transcranial magnetic stimulation (TMS)/tDCS combined protocol. Motor evoked potentials (MEPs) together with vital parameters [e.g., blood pressure, heart-rate variability (HRV), and sympathovagal balance] were recorded and monitored before, during, and after anodal, cathodal, or sham tDCS. Ten MEPs, every 2.5-min time windows, were recorded from the right first dorsal interosseous (FDI), while 5-min epochs were used to record vital parameters. The protocol included 15 min of pre-tDCS and of online tDCS (anodal, cathodal, or sham). After-effects were recorded for 30 min. We showed a polarity-independent stabilization of cortical excitability level, a polarity-specific after-effect for cathodal and anodal stimulation, and an absence of persistent excitability changes during online stimulation. No significant effects on vital parameters emerged both during and after tDCS, while a linear increase in systolic/diastolic blood pressure and HRV was observed during each tDCS condition, as a possible unspecific response to experimental demands. Taken together, current findings provide new insights on the safety of monopolar tDCS, promoting its application both in research and clinical settings.
Keywords: neuromodulation; safety; transcranial direct current stimulation; transcranial magnetic stimulation; vital parameters.
Figures

Similar articles
-
Transcranial direct-current stimulation reduced the excitability of diaphragmatic corticospinal pathways whatever the polarity used.Respir Physiol Neurobiol. 2013 Oct 1;189(1):183-7. doi: 10.1016/j.resp.2013.07.024. Epub 2013 Aug 7. Respir Physiol Neurobiol. 2013. PMID: 23933029 Clinical Trial.
-
Tracking the Effect of Cathodal Transcranial Direct Current Stimulation on Cortical Excitability and Connectivity by Means of TMS-EEG.Front Neurosci. 2018 May 15;12:319. doi: 10.3389/fnins.2018.00319. eCollection 2018. Front Neurosci. 2018. PMID: 29867330 Free PMC article.
-
Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach.Neuroimage. 2013 Dec;83:569-80. doi: 10.1016/j.neuroimage.2013.06.076. Epub 2013 Jul 9. Neuroimage. 2013. PMID: 23845429
-
Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants.Physiol Rep. 2016 Aug;4(15):e12884. doi: 10.14814/phy2.12884. Physiol Rep. 2016. PMID: 27495298 Free PMC article.
-
The effects of transcranial direct current stimulation on corticospinal excitability: A systematic review of nonsignificant findings.Eur J Neurosci. 2023 Aug;58(4):3074-3097. doi: 10.1111/ejn.16073. Epub 2023 Jul 5. Eur J Neurosci. 2023. PMID: 37407275 Review.
Cited by
-
Frequency-specific insight into short-term memory capacity.J Neurophysiol. 2016 Jul 1;116(1):153-8. doi: 10.1152/jn.01080.2015. Epub 2016 Apr 27. J Neurophysiol. 2016. PMID: 27121583 Free PMC article.
-
Does M1 anodal transcranial direct current stimulation affects online and offline motor learning in patients with multiple sclerosis?Neurol Sci. 2020 Sep;41(9):2539-2546. doi: 10.1007/s10072-020-04359-9. Epub 2020 Mar 27. Neurol Sci. 2020. PMID: 32219594 Clinical Trial.
-
Strengthening of Existing Episodic Memories Through Non-invasive Stimulation of Prefrontal Cortex in Older Adults with Subjective Memory Complaints.Front Aging Neurosci. 2017 Dec 5;9:401. doi: 10.3389/fnagi.2017.00401. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29259554 Free PMC article.
-
Older adults get episodic memory boosting from noninvasive stimulation of prefrontal cortex during learning.Neurobiol Aging. 2016 Mar;39:210-216. doi: 10.1016/j.neurobiolaging.2015.12.010. Epub 2015 Dec 29. Neurobiol Aging. 2016. PMID: 26923418 Free PMC article. Clinical Trial.
-
A scoping review of neuromodulation techniques for controlling blood pressure: what are the ups and downs to this approach?Bioelectron Med. 2025 Aug 15;11(1):19. doi: 10.1186/s42234-025-00181-w. Bioelectron Med. 2025. PMID: 40814063 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

