SCF(SLF)-mediated cytosolic degradation of S-RNase is required for cross-pollen compatibility in S-RNase-based self-incompatibility in Petunia hybrida
- PMID: 25101113
- PMCID: PMC4106197
- DOI: 10.3389/fgene.2014.00228
SCF(SLF)-mediated cytosolic degradation of S-RNase is required for cross-pollen compatibility in S-RNase-based self-incompatibility in Petunia hybrida
Abstract
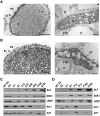
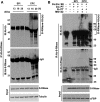
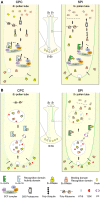
Many flowering plants adopt self-incompatibility (SI) to maintain their genetic diversity. In species of Solanaceae, Plantaginaceae, and Rosaceae, SI is genetically controlled by a single S-locus with multiple haplotypes. The S-locus has been shown to encode S-RNases expressed in pistil and multiple SLF (S-locus F-box) proteins in pollen controlling the female and male specificity of SI, respectively. S-RNases appear to function as a cytotoxin to reject self-pollen. In addition, SLFs have been shown to form SCF (SKP1/Cullin1/F-box) complexes to serve as putative E3 ubiquitin ligase to interact with S-RNases. Previously, two different mechanisms, the S-RNase degradation and the S-RNase compartmentalization, have been proposed as the restriction mechanisms of S-RNase cytotoxicity allowing compatible pollination. In this study, we have provided several lines of evidence in support of the S-RNase degradation mechanism by a combination of cellular, biochemical and molecular biology approaches. First, both immunogold labeling and subcellular fractionation assays showed that two key pollen SI factors, PhS3L-SLF1 and PhSSK1 (SLF-interacting SKP1-like1) from Petunia hybrida, a Solanaceous species, are co-localized in cytosols of both pollen grains and tubes. Second, PhS3L-RNases are mainly detected in the cytosols of both self and non-self-pollen tubes after pollination. Third, we found that PhS-RNases selectively interact with PhSLFs by yeast two-hybrid and co-immunoprecipitation assays. Fourth, S-RNases are specifically degraded in compatible pollen tubes by non-self SLF action. Taken together, our results demonstrate that SCF(SLF-mediated) non-self S-RNase degradation occurs in the cytosol of pollen tube through the ubiquitin/26S proteasome system serving as the major mechanism to neutralize S-RNase cytotoxicity during compatible pollination in P. hybrida.
Keywords: S-RNase localization; SCFSLF; cross-pollen compatibility; self-incompatibility; self-pollen incompatibility; ubiquitin/26S proteasome system.
Figures



References
-
- Arrese E. L., Gazard J. L., Flowers M. T., Soulages J. L., Wells M. A. (2001). Diacylglycerol transport in the insect fat body: evidence of involvement of lipid droplets and the cytosolic fraction. Lipid Res. 42, 225–234 - PubMed
-
- Busot G. Y., McClure B., Ibarra-Sánchez C. P., Jiménez-Durán K., Vázquez-Santana S., Cruz-García F. (2008). Pollination in Nicotiana alata stimulates synthesis and transfer to the stigmatic surface of NaStEP, a vacuolar Kunitz proteinase inhibitor homologue. J. Exp. Bot. 59, 3187–3201 10.1093/jxb/ern175 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

