A new model for providing cell-free DNA and risk assessment for chromosome abnormalities in a public hospital setting
- PMID: 25101177
- PMCID: PMC4102090
- DOI: 10.1155/2014/962720
A new model for providing cell-free DNA and risk assessment for chromosome abnormalities in a public hospital setting
Abstract
Objective: Cell-free DNA (cfDNA) offers highly accurate noninvasive screening for Down syndrome. Incorporating it into routine care is complicated. We present our experience implementing a novel program for cfDNA screening, emphasizing patient education, genetic counseling, and resource management.
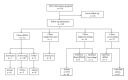
Study design: Beginning in January 2013, we initiated a new patient care model in which high-risk patients for aneuploidy received genetic counseling at 12 weeks of gestation. Patients were presented with four pathways for aneuploidy risk assessment and diagnosis: (1) cfDNA; (2) integrated screening; (3) direct-to-invasive testing (chorionic villus sampling or amniocentesis); or (4) no first trimester diagnostic testing/screening. Patients underwent follow-up genetic counseling and detailed ultrasound at 18-20 weeks to review first trimester testing and finalize decision for amniocentesis.
Results: Counseling and second trimester detailed ultrasound were provided to 163 women. Most selected cfDNA screening (69%) over integrated screening (0.6%), direct-to-invasive testing (14.1%), or no screening (16.6%). Amniocentesis rates decreased following implementation of cfDNA screening (19.0% versus 13.0%, P < 0.05).
Conclusion: When counseled about screening options, women often chose cfDNA over integrated screening. This program is a model for patient-directed, efficient delivery of a newly available high-level technology in a public health setting. Genetic counseling is an integral part of patient education and determination of plan of care.
Figures
Similar articles
-
Clinical implementation of routine screening for fetal trisomies in the UK NHS: cell-free DNA test contingent on results from first-trimester combined test.Ultrasound Obstet Gynecol. 2016 Jan;47(1):45-52. doi: 10.1002/uog.15783. Epub 2015 Oct 26. Ultrasound Obstet Gynecol. 2016. PMID: 26498918
-
Society for Maternal-Fetal Medicine Consult Series #57: Evaluation and management of isolated soft ultrasound markers for aneuploidy in the second trimester: (Replaces Consults #10, Single umbilical artery, October 2010; #16, Isolated echogenic bowel diagnosed on second-trimester ultrasound, August 2011; #17, Evaluation and management of isolated renal pelviectasis on second-trimester ultrasound, December 2011; #25, Isolated fetal choroid plexus cysts, April 2013; #27, Isolated echogenic intracardiac focus, August 2013).Am J Obstet Gynecol. 2021 Oct;225(4):B2-B15. doi: 10.1016/j.ajog.2021.06.079. Epub 2021 Jun 23. Am J Obstet Gynecol. 2021. PMID: 34171388
-
Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result.Ultrasound Obstet Gynecol. 2016 Jun;47(6):698-704. doi: 10.1002/uog.15851. Epub 2016 Apr 25. Ultrasound Obstet Gynecol. 2016. PMID: 26743020
-
Screening for fetal aneuploidy.Semin Perinatol. 2016 Feb;40(1):35-43. doi: 10.1053/j.semperi.2015.11.006. Epub 2015 Dec 25. Semin Perinatol. 2016. PMID: 26725144 Review.
-
Prenatal diagnosis for detection of aneuploidy: the options.Radiol Clin North Am. 2003 Jul;41(4):695-708. doi: 10.1016/s0033-8389(03)00044-7. Radiol Clin North Am. 2003. PMID: 12899486 Review.
Cited by
-
Economic Impact of Coverage Expansion for Non-invasive Prenatal Testing Through a Performance-Based Risk-Sharing Agreement.Pharmacoecon Open. 2021 Sep;5(3):449-458. doi: 10.1007/s41669-021-00261-y. Epub 2021 Mar 10. Pharmacoecon Open. 2021. PMID: 33689154 Free PMC article.
-
An Economic Analysis of Cell-Free DNA Non-Invasive Prenatal Testing in the US General Pregnancy Population.PLoS One. 2015 Jul 9;10(7):e0132313. doi: 10.1371/journal.pone.0132313. eCollection 2015. PLoS One. 2015. PMID: 26158465 Free PMC article.
-
Pre- and post-test genetic counseling for chromosomal and Mendelian disorders.Semin Perinatol. 2016 Feb;40(1):44-55. doi: 10.1053/j.semperi.2015.11.007. Epub 2015 Dec 21. Semin Perinatol. 2016. PMID: 26718445 Free PMC article. Review.
-
Trial by Dutch laboratories for evaluation of non-invasive prenatal testing. Part I-clinical impact.Prenat Diagn. 2016 Dec;36(12):1083-1090. doi: 10.1002/pd.4945. Epub 2016 Nov 15. Prenat Diagn. 2016. PMID: 27750376 Free PMC article.
References
-
- Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. American Journal of Obstetrics and Gynecology. 2011;204(3):205–e11. - PubMed
-
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genetics in Medicine. 2011;13(11):913–920. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical