Role of Th17 Cells in the Pathogenesis of Human IBD
- PMID: 25101191
- PMCID: PMC4005031
- DOI: 10.1155/2014/928461
Role of Th17 Cells in the Pathogenesis of Human IBD
Abstract
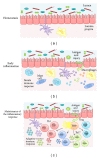
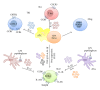
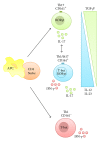
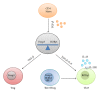
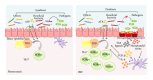
The gastrointestinal tract plays a central role in immune system, being able to mount efficient immune responses against pathogens, keeping the homeostasis of the human gut. However, conditions like Crohn's disease (CD) or ulcerative colitis (UC), the main forms of inflammatory bowel diseases (IBD), are related to an excessive and uncontrolled immune response against normal microbiota, through the activation of CD4(+) T helper (Th) cells. Classically, IBD was thought to be primarily mediated by Th1 cells in CD or Th2 cells in UC, but it is now known that Th17 cells and their related cytokines are crucial mediators in both conditions. Th17 cells massively infiltrate the inflamed intestine of IBD patients, where they produce interleukin- (IL-) 17A and other cytokines, triggering and amplifying the inflammatory process. However, these cells show functional plasticity, and they can be converted into either IFN- γ producing Th1 cells or regulatory T cells. This review will summarize the current knowledge regarding the regulation and functional role of Th17 cells in the gut. Deeper insights into their plasticity in inflammatory conditions will contribute to advancing our understanding of the mechanisms that regulate mucosal homeostasis and inflammation in the gut, promoting the design of novel therapeutic approaches for IBD.
Figures
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. - PubMed
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nature Reviews Immunology. 2003;3(4):331–341. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology. 2003;3(7):521–533. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

