Therapy of hypoparathyroidism by replacement with parathyroid hormone
- PMID: 25101193
- PMCID: PMC4102094
- DOI: 10.1155/2014/765629
Therapy of hypoparathyroidism by replacement with parathyroid hormone
Abstract
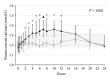
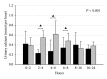
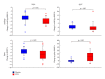
Hypoparathyroidism (HypoPT) is a state of hypocalcemia due to inappropriate low levels of parathyroid hormone (PTH). HypoPT is normally treated by calcium supplements and activated vitamin D analogues. Although plasma calcium is normalized in response to conventional therapy, quality of life (QoL) seems impaired and patients are at increased risk of renal complications. A number of studies have suggested subcutaneous injections with PTH as an alternative therapy. By replacement with the missing hormone, urinary calcium may be lowered and QoL may improve. PTH replacement therapy (PTH-RT) possesses, nevertheless, a number of challenges. If PTH is injected only once a day, fluctuations in calcium levels may occur resulting in hypercalcemia in the hours following an injection. Twice-a-day injections seem to cause less fluctuation in plasma calcium but do stimulate bone turnover to above normal. Most recently, continuous delivery of PTH by pump has appeared as a feasible alternative to injections. Plasma calcium levels do not fluctuate, urinary calcium is lowered, and bone turnover is only stimulated modestly (into the normal range). Further studies are needed to assess the long-term effects. If beneficial, it seems likely that standard treatment of HypoPT in the future will change into replacement therapy with the missing hormone.
Figures

References
-
- Thakker RV. Genetic developments in hypoparathyroidism. The Lancet. 2001;357(9261):974–976. - PubMed
-
- Lima K, Abrahamsen TG, Wolff AB, et al. Hypoparathyroidism and autoimmunity in the 22q11.2 deletion syndrome. European Journal of Endocrinology. 2011;165(2):345–352. - PubMed
-
- Al-Azem H, Khan AA. Hypoparathyroidism. Best Practice and Research: Clinical Endocrinology and Metabolism. 2012;26(4):517–522. - PubMed
-
- Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. Journal of Bone and Mineral Research. 2013;28(11):2277–2285. - PubMed
-
- Clarke B, Leibson CL, Emerson J, Ransom JC, Lagast H. Co-morbid medical conditions associated with prevalent hypoparathyroidism: a population-based. Journal of Bone and Mineral Research. 2011;26(supplement 1) http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=9981....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

