Emerging insights into barriers to effective brain tumor therapeutics
- PMID: 25101239
- PMCID: PMC4104487
- DOI: 10.3389/fonc.2014.00126
Emerging insights into barriers to effective brain tumor therapeutics
Abstract
There is great promise that ongoing advances in the delivery of therapeutics to the central nervous system (CNS) combined with rapidly expanding knowledge of brain tumor patho-biology will provide new, more effective therapies. Brain tumors that form from brain cells, as opposed to those that come from other parts of the body, rarely metastasize outside of the CNS. Instead, the tumor cells invade deep into the brain itself, causing disruption in brain circuits, blood vessel and blood flow changes, and tissue swelling. Patients with the most common and deadly form, glioblastoma (GBM) rarely live more than 2 years even with the most aggressive treatments and often with devastating neurological consequences. Current treatments include maximal safe surgical removal or biopsy followed by radiation and chemotherapy to address the residual tumor mass and invading tumor cells. However, delivering effective and sustained treatments to these invading cells without damaging healthy brain tissue is a major challenge and focus of the emerging fields of nanomedicine and viral and cell-based therapies. New treatment strategies, particularly those directed against the invasive component of this devastating CNS disease, are sorely needed. In this review, we (1) discuss the history and evolution of treatments for GBM, (2) define and explore three critical barriers to improving therapeutic delivery to invasive brain tumors, specifically, the neuro-vascular unit as it relates to the blood brain barrier, the extra-cellular space in regard to the brain penetration barrier, and the tumor genetic heterogeneity and instability in association with the treatment efficacy barrier, and (3) identify promising new therapeutic delivery approaches that have the potential to address these barriers and create sustained, meaningful efficacy against GBM.
Keywords: advanced therapeutics; blood brain barrier; brain cancer; drug delivery; glioblastoma; immunotherapy; nanomedicine; nanotechnology.
Figures

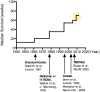
 ). More recently, combination chemotherapy regimens have been suggested to increase this median survival upwards of 20 months.
). More recently, combination chemotherapy regimens have been suggested to increase this median survival upwards of 20 months.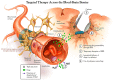
References
-
- McComb RD, Burger PC. Pathologic analysis of primary brain tumors. Neurol Clin (1985) 3(4):711–28 - PubMed
-
- Gruber ML, Hochberg FH. Systematic evaluation of primary brain tumors. J Nucl Med (1990) 31(6):969–71 - PubMed
-
- Dandy W. Removal of right cerebral hemisphere for certain tumors with hemiplegia. J Am Med Assoc (1928) 90:823–5 10.1001/jama.1928.02690380007003 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

