SMAC Mimetic BV6 Enables Sensitization of Resistant Tumor Cells but also Affects Cytokine-Induced Killer (CIK) Cells: A Potential Challenge for Combination Therapy
- PMID: 25101252
- PMCID: PMC4103003
- DOI: 10.3389/fped.2014.00075
SMAC Mimetic BV6 Enables Sensitization of Resistant Tumor Cells but also Affects Cytokine-Induced Killer (CIK) Cells: A Potential Challenge for Combination Therapy
Abstract
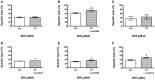
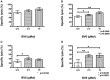
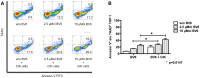
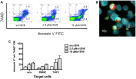
Allogeneic hematopoietic stem cell transplantation (HSCT) is an established treatment option for high-risk hematological malignancies, and may also be offered to patients with solid malignancies refractory to conventional therapies. In case of patients' relapse, refractory tumor cells may then be targeted by cellular therapy-based combination strategies. Here, we investigated the potential of small molecule IAP (SMAC mimetic) BV6 in increasing cytokine-induced killer (CIK) cell-mediated cytotoxicity against different tumor targets. Four-hour pre-incubation with 2.5 μMol BV6 moderately enhanced CIK cell-mediated lysis of hematological (H9, THP-1, and Tanoue) and solid malignancies (RH1, RH30, and TE671). However, BV6 also increased apoptosis of non-malignant cells like peripheral blood mononuclear cells and most notably had an inhibitory effect on immune cells potentially limiting their cytotoxic potential. Hence, cytotoxicity increased in a dose-dependent manner when BV6 was removed before CIK cells were added to tumor targets. However, cytotoxic potential was not further increasable by extending BV6 pre-incubation period of target cells from 4 to 12 h. Molecular studies revealed that BV6 sensitization of target cells involved activation of caspases. Here, we provide evidence that SMAC mimetic may sensitize targets cells for CIK cell-induced cell death. However, BV6 also increased apoptosis of non-malignant cells like CIK cells and peripheral mononuclear cells. These findings may therefore be important for cell- and small molecule IAP-based combination therapies of resistant cancers after allogeneic HSCT.
Keywords: BV6; CIK cells; cellular therapy; leukemia; tumors.
Figures


Similar articles
-
Cooperative TRAIL production mediates IFNα/Smac mimetic-induced cell death in TNFα-resistant solid cancer cells.Oncotarget. 2016 Jan 26;7(4):3709-25. doi: 10.18632/oncotarget.6915. Oncotarget. 2016. PMID: 26788912 Free PMC article.
-
Smac mimetic induces cell death in a large proportion of primary acute myeloid leukemia samples, which correlates with defined molecular markers.Oncotarget. 2016 Aug 2;7(31):49539-49551. doi: 10.18632/oncotarget.10390. Oncotarget. 2016. PMID: 27385100 Free PMC article.
-
Characterization of BV6-Induced Sensitization to the NK Cell Killing of Pediatric Rhabdomyosarcoma Spheroids.Cells. 2023 Mar 15;12(6):906. doi: 10.3390/cells12060906. Cells. 2023. PMID: 36980247 Free PMC article.
-
Cytokine-Induced Killer Cells: A Unique Platform for Adoptive Cell Immunotherapy after Allogeneic Hematopoietic Stem Cell Transplantation.Transfus Med Hemother. 2024 Sep 24;52(1):77-95. doi: 10.1159/000540964. eCollection 2025 Feb. Transfus Med Hemother. 2024. PMID: 39944412 Free PMC article. Review.
-
Cytokine-induced killer cells: NK-like T cells with cytotolytic specificity against leukemia.Leuk Lymphoma. 2003 Sep;44(9):1457-62. doi: 10.3109/10428190309178764. Leuk Lymphoma. 2003. PMID: 14565644 Review.
Cited by
-
The Smac Mimetic BV6 Improves NK Cell-Mediated Killing of Rhabdomyosarcoma Cells by Simultaneously Targeting Tumor and Effector Cells.Front Immunol. 2017 Mar 7;8:202. doi: 10.3389/fimmu.2017.00202. eCollection 2017. Front Immunol. 2017. PMID: 28326081 Free PMC article.
-
Targeting ubiquitin signaling for cancer immunotherapy.Signal Transduct Target Ther. 2021 Jan 13;6(1):16. doi: 10.1038/s41392-020-00421-2. Signal Transduct Target Ther. 2021. PMID: 33436547 Free PMC article. Review.
-
Clinical outcome of immunotherapy with dendritic cell vaccine and cytokine-induced killer cell therapy in hepatobiliary and pancreatic cancer.Mol Clin Oncol. 2016 Jan;4(1):129-133. doi: 10.3892/mco.2015.660. Epub 2015 Oct 23. Mol Clin Oncol. 2016. PMID: 26870371 Free PMC article.
-
Potency and Selectivity of SMAC/DIABLO Mimetics in Solid Tumor Therapy.Cells. 2020 Apr 18;9(4):1012. doi: 10.3390/cells9041012. Cells. 2020. PMID: 32325691 Free PMC article. Review.
-
Potentiation of apoptosis in drug-resistant mantle cell lymphoma cells by MCL-1 inhibitor involves downregulation of inhibitor of apoptosis proteins.Cell Death Dis. 2023 Nov 2;14(11):714. doi: 10.1038/s41419-023-06233-w. Cell Death Dis. 2023. PMID: 37919300 Free PMC article.
References
-
- Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood (2011) 118:5681–810.1182/blood-2011-04-348805 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

