Human isoprenoid synthase enzymes as therapeutic targets
- PMID: 25101260
- PMCID: PMC4106277
- DOI: 10.3389/fchem.2014.00050
Human isoprenoid synthase enzymes as therapeutic targets
Abstract
In the human body, the complex biochemical network known as the mevalonate pathway is responsible for the biosynthesis of all isoprenoids, which consists of a vast array of metabolites that are vital for proper cellular functions. Two key isoprenoids, farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) are responsible for the post-translational prenylation of small GTP-binding proteins, and serve as the biosynthetic precursors to numerous other biomolecules. The down-stream metabolite of FPP and GGPP is squalene, the precursor to steroids, bile acids, lipoproteins, and vitamin D. In the past, interest in prenyl synthase inhibitors focused mainly on the role of the FPP in lytic bone diseases. More recently pre-clinical and clinical studies have strongly implicated high levels of protein prenylation in a plethora of human diseases, including non-skeletal cancers, the progression of neurodegenerative diseases and cardiovascular diseases. In this review, we focus mainly on the potential therapeutic value of down-regulating the biosynthesis of FPP, GGPP, and squalene. We summarize the most recent drug discovery efforts and the structural data available that support the current on-going studies.
Keywords: farnesyl pyrophosphate; geranylgeranyl pyrophosphate; isoprenoids; mevalonate pathway; squalene.
Figures
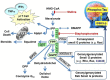
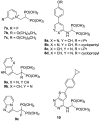
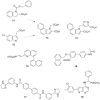
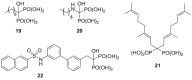
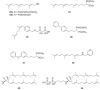
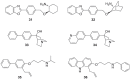
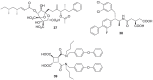
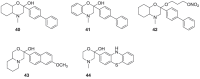
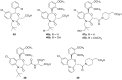
 ). FPPS, GGPS, and SQS enzymes are highlighted in green color boxes; other key enzymes are highlighted in gray color boxes.
). FPPS, GGPS, and SQS enzymes are highlighted in green color boxes; other key enzymes are highlighted in gray color boxes.


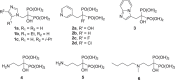
References
-
- Amin D., Rutledge R. Z., Needle S. N., Galczenski H. F., Neuenschwander K., Scotese A. C., et al. (1997). RPR 107393, a potent squalene synthase inhibitor and orally effective cholesterol-lowering agent: comparison with inhibitors of HMG-CoA reductase. J. Pharmacol. Exp. Ther. 281, 746–752 - PubMed
-
- Amstutz R., Bold G., Cotesta S., Jahnke W., Marzinzik A., Mueller-Hartwieg C., et al. (2009). Quinolines as Inhibitors of Farnesyl Pyrophosphate Synthase. WO 2009/106586 A1
-
- Baxter A., Fitzgerald B. J., Hutson J. L., McCarthy A. D., Motteram J. M., Ross B. C., et al. (1992). Squalestatin 1, a potent inhibitor of squalene synthase, which lowers serum cholesterol in vivo. J. Biol. Chem. 267, 11705–11708 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

