Immediate postabortal insertion of intrauterine devices
- PMID: 25101364
- PMCID: PMC7079711
- DOI: 10.1002/14651858.CD001777.pub4
Immediate postabortal insertion of intrauterine devices
Abstract
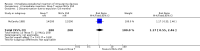
Background The use of an effective contraceptive may be necessary after an abortion. Insertion of an intrauterine device (IUD) may be done the same day or later. Immediate IUD insertion is an option since the woman is not pregnant, pain of insertion is less because the cervical os is open, and her motivation to use contraception may be high. However, insertion of an IUD immediately after a pregnancy ends carries risks, such as spontaneous expulsion.Objectives To assess the safety and efficacy of IUD insertion immediately after spontaneous or induced abortion.Search methods We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, POPLINE, ClinicalTrials.gov,and ICTRP in January 27, 2014. We also contacted investigators to identify other trials.Selection criteria We sought all randomised controlled trials (RCTs) with at least one treatment arm that involved IUD insertion immediately after an induced abortion or after curettage for spontaneous abortion.Data collection and analysis We evaluated the methodological quality of each report and abstracted the data. We focused on discontinuation rates for accidental pregnancy, perforation, expulsion, and pelvic inflammatory disease.We computed the weighted average of the rate ratios.We compute drisk ratios (RRs) with 95% Confidence Intervals (CIs).We performed an intention-to-treat (ITT) analysis by including all randomised participants in the analysis according to the Cochrane Handbook for Systematic Reviews of Interventions.Main results We identified 12 trials most of which are of moderate risk of bias involving 7,119 participants which described random assignment.Five trials randomised to either immediate or delayed insertion of IUD. One of them randomised to immediate versus delayed insertion of Copper 7 showed immediate insertion of the Copper 7 was associated with a higher risk of expulsion than was delayed insertion(RR 11.98, 95% CI 1.61 to 89.35,1 study, 259 participants); the quality of evidence was moderate. Moderate quality of evidence also suggests that use and expulsion of levonorgestrel-releasing intrauterine system or CuT380A was more likely for immediate compared to delayed insertion risk ratio (RR) 1.40 (95% CI 1.24 to 1.58; 3 studies; 878 participants) and RR 2.64 ( 95% CI 1.16 to 6.00; 3 studies; 878 participants) respectively. Another trial randomised to the levonorgestrel IUD or Nova T showed discontinuation rates due to pregnancy were likely to be higher for women in the Nova T group. (MD 8.70, 95% CI 3.92 to 13.48;1 study; 438 participants);moderate quality evidence.Seven trials examined immediate insertion of IUD only. From meta-analysis of two multicentre trials, pregnancy was less likely for the TCu 220C versus the Lippes Loop (RR 0.43, 95% CI 0.24 to 0.75; 2 studies; 2257 participants ) as was expulsion (RR 0.61, 95% CI0.46 to 0.81; 2 studies; 2257 participants). Estimates for the TCu 220 versus the Copper 7 were RR 0.42 ( 95% CI 0.23 to 0.77; 2 studies, 2,274 participants) and RR 0.68, (95% CI 0.51 to 0.91); 2 studies, 2,274 participants), respectively. In other work, adding copper sleeves to the Lippes Loop improved efficacy (RR 3.40, 95% CI 1.28 to 9.04, 1 study, 400 participants) and reduced expulsion(RR 3.00, 95% CI 1.51 to 5.97; 1 study, 400 participants).Authors' conclusions Moderate quality evidence shows that insertion of an IUD immediately after abortion is safe and practical. IUD expulsion rates appear higher immediately after abortions compared to delayed insertions. However, at six months postabortion, IUD use is higher following immediate insertion compared to delayed insertion.
Conflict of interest statement
None known for this update.
Figures
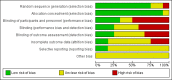






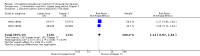




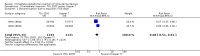
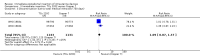

























Update of
-
Immediate postabortal insertion of intrauterine devices.Cochrane Database Syst Rev. 2010 Jun 16;(6):CD001777. doi: 10.1002/14651858.CD001777.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 Jul 28;(7):CD001777. doi: 10.1002/14651858.CD001777.pub4. PMID: 20556754 Updated.
Similar articles
-
Immediate postabortal insertion of intrauterine devices.Cochrane Database Syst Rev. 2010 Jun 16;(6):CD001777. doi: 10.1002/14651858.CD001777.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 Jul 28;(7):CD001777. doi: 10.1002/14651858.CD001777.pub4. PMID: 20556754 Updated.
-
Immediate postabortal insertion of intrauterine devices.Cochrane Database Syst Rev. 2002;(3):CD001777. doi: 10.1002/14651858.CD001777. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001777. doi: 10.1002/14651858.CD001777.pub2. PMID: 12137634 Updated.
-
Immediate postabortal insertion of intrauterine devices.Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001777. doi: 10.1002/14651858.CD001777.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2010 Jun 16;(6):CD001777. doi: 10.1002/14651858.CD001777.pub3. PMID: 15495018 Updated.
-
Immediate post-abortal insertion of intrauterine devices.Cochrane Database Syst Rev. 2000;(2):CD001777. doi: 10.1002/14651858.CD001777. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2002;(3):CD001777. doi: 10.1002/14651858.CD001777. PMID: 10796820 Updated.
-
Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception.Cochrane Database Syst Rev. 2022 Oct 27;10(10):CD011913. doi: 10.1002/14651858.CD011913.pub3. Cochrane Database Syst Rev. 2022. PMID: 36302159 Free PMC article.
Cited by
-
Perception And Use Of Intrauterine Contraceptive Devices (IUCD) Among Married Women Of Reproductive Age In Bhaktapur, Nepal.Open Access J Contracept. 2019 Nov 28;10:69-77. doi: 10.2147/OAJC.S219188. eCollection 2019. Open Access J Contracept. 2019. PMID: 31819678 Free PMC article.
-
[Preventive activities in women. PAPPS update 2022].Aten Primaria. 2022 Oct;54 Suppl 1(Suppl 1):102471. doi: 10.1016/j.aprim.2022.102471. Aten Primaria. 2022. PMID: 36435585 Free PMC article. Spanish.
-
Actividades preventivas en la mujer. Actualización PAPPS 2018.Aten Primaria. 2018 May;50 Suppl 1(Suppl 1):125-146. doi: 10.1016/S0212-6567(18)30366-4. Aten Primaria. 2018. PMID: 29866353 Free PMC article. Spanish. No abstract available.
-
One-year follow up of contraceptive use and pregnancy rates after early medical abortion: Secondary outcomes from a randomized controlled trial of immediate post-abortion placement of intrauterine devices.Acta Obstet Gynecol Scand. 2023 Dec;102(12):1694-1702. doi: 10.1111/aogs.14662. Epub 2023 Aug 23. Acta Obstet Gynecol Scand. 2023. PMID: 37614066 Free PMC article. Clinical Trial.
-
Breastfeeding and contraception counseling: a qualitative study.BMC Pregnancy Childbirth. 2022 Feb 25;22(1):154. doi: 10.1186/s12884-022-04451-2. BMC Pregnancy Childbirth. 2022. PMID: 35216562 Free PMC article.
References
References to studies included in this review
Bednarek 2011 {published data only}
-
- Bednarek PA, Creinin MD, Reeves MF, Cwiak CA, Espey E, Jensen JT, Post‐Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. The New England Journal of Medicine 2011;364(23):2208‐17. [NCT00562276] - PubMed
Cremer 2011 {published data only}
Gillett 1980 {published and unpublished data}
-
- Gillett PG, Lee NH, Yuzpe AA, Cerskus I. A comparison of the efficacy and acceptability of the Copper‐7 intrauterine device following immediate or delayed insertion after first‐trimester therapeutic abortion. Fertility and Sterility 1980;34(2):121‐4. - PubMed
Hohmann 2012 {published data only}
-
- Hohmann HL, Reeves MF, Chen BA, Perriera L, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestrel‐releasing intrauterine device following dilation and evacuation: a randomized controlled trial. Contraception 2012;85(3):240‐5. [DOI: 10.1016/j.contraception.2011.08.002; NCT00475228] - DOI - PubMed
Lim 1985 {published and unpublished data}
-
- Lim LS, McCarthy T, Yong YM, Ratnam SS. Post‐abortion insertion of MLCu 250 and MLCu 375 ‐ a comparative trial. Contraception 1985;31(5):471‐7. - PubMed
McCarthy 1985 {published and unpublished data}
-
- McCarthy T, Ramachandran L, Huang HS, Ratnam S. Postabortion insertion of the Nova T and MLCu250: preliminary results of a comparative study. Advances in Contraception 1985;1(2):161‐5. - PubMed
Nielsen 1984 {published data only}
-
- Nielsen NC, Nygren K‐G, Allonen H. Three years of experience after post‐abortal insertion of Nova‐T and Copper‐T‐200. Acta Obstetricia et Gynecologica Scandinavica 1984;63(3):261‐4. - PubMed
Pakarinen 2003 {published and unpublished data}
-
- Luukkainen T, Allonen H, Haukkamaa M, Holma P, Pyorala T, Terho J, et al. Effective contraception with the levonorgestrel‐releasing intrauterine device: 12‐month report of a European multicenter study. Contraception 1987;36(2):169‐79. - PubMed
-
- Pakarinen P, Toivonen J, Luukkainen T. Randomized comparison of levonorgestrel‐ and copper‐releasing intrauterine systems immediately after abortion, with 5 years' follow‐up. Contraception 2003;68(1):31‐4. - PubMed
-
- Suvisaari J, Lahteenmaki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel‐releasing intrauterine system. Contraception 1996;54(4):201‐8. - PubMed
Randic 1983 {published and unpublished data}
-
- Randic L, Balogh SA. The effect of hydrogel on the reduction of bleeding associated with IUD use: a comparative study of the plain and Hydron‐coated Spring Coil. Contraceptive Delivery Systems 1983;4(4):301‐10. - PubMed
Randic 1991 {published and unpublished data}
-
- Randic L, Haller H, Perovic M, Farr G. The effect of adding copper onto Lippes Loop IUDs: results from a ten‐year study in Yugoslavia. Contraception 1991;43(3):229‐39. - PubMed
WHO 1983a {published and unpublished data}
-
- World Health Organization Task Force on Intrauterine Devices for Fertility Regulation. IUD insertion following termination of pregnancy: a clinical trial of the TCu 220C, Lippes Loop D, and Copper 7. Studies in Family Planning 1983;14(4):99‐108. - PubMed
WHO 1983b {published data only}
-
- World Health Organization Task Force on Intrauterine Devices for Fertility Regulation. IUD insertion following spontaneous abortion: a clinical trial of the TCu 220C, Lippes Loop D, and Copper 7. Studies in Family Planning 1983;14(4):109‐14. - PubMed
References to studies excluded from this review
Chowdhury 1979 {published data only}
-
- Chowdhury NNR, Mandal GS, Das M. Comparative study of Lippes Loop and CuT inserted in immediate post‐abortal period. Journal of Obstetrics and Gynaecology of India 1979;29(2):239‐44. - PubMed
Goldsmith 1972 {published data only}
-
- Goldsmith A, Goldberg R, Eyzaguirre H, Lizana L. Immediate postabortal intrauterine contraceptive device insertion: a double‐blind study. American Journal of Obstetrics and Gynecology 1972;112(7):957‐62. - PubMed
NCT00737178 {published data only}
-
- Davis A. Immediate versus delayed insertion of the copper intrauterine device (IUD) after medication abortion. www.clinicaltrials.gov/ct2/show/NCT00737178 (accessed 03 Feb 2010).
Querido 1985 {published data only}
-
- Querido L, Ketting E, Haspels AA. IUD insertion following induced abortion. Contraception 1985;31(6):603‐10. - PubMed
References to ongoing studies
[ISRCTN: 19506752] {published data only}
NCT00877344 {published data only}
-
- Norman WV. Better contraceptive choices: immediate or delayed insertion of IUD after second trimester abortion. www.clinicaltrials.gov/ct2/show/NCT00877344 (accessed 03 Feb 2010).
Additional references
Burkman 2007
-
- Burkman RT. Contraception & Family Planning. In: Decherney AH, Nathan L, Goodwin TM, Laufer N editor(s). Current Diagnosis & Treatment. Obstetrics & Gynecology. 10th Edition. New York, USA: McGraw‐Hill Companies, 2007:579‐597.
Drey 2009
-
- Drey EA, Reeves MF, Ogawa DD, Sokoloff A, Darney PD, Steinauer JE. Insertion of intrauterine contraceptives immediately following first‐ and second‐trimester abortions. Contraception 2009;79(5):397‐402. - PubMed
Farley 1992
-
- Farley TMM, Rowe PJ, Meirik O, Rosenberg MJ, Chen J‐H. IUDs and pelvic inflammatory disease. Lancet 1992;340(8813):248‐9. - PubMed
Goodman 2008
-
- Goodman S, Hendlish SK, Reeves MF, Foster‐Rosales A. Impact of immediate postabortal insertion of intrauterine contraception on repeat abortion. Contraception 2008;78(2):143‐8. - PubMed
Grimes 2008
-
- Grimes DA, Mishell DR. Intrauterine contraception as an alternative to interval tubal sterilization. Contraception 2008;77(1):6‐9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Available from www.cochrane‐handbook.org [updated March 2011]; Vol. Version 5.1.0.
Mahomed 1997
-
- Mahomed K, Healy J, Tandom S. Family planning counselling ‐ a priority for post abortion care. Central African Journal of Medicine 1997;43(7):205‐7. - PubMed
McLaurin 1993
-
- McLaurin KE, Senanayake P, Toubia N, Ladipo OA. Post‐abortion family planning: reversing a legacy of neglect. Lancet 1993;342(8879):1099‐100. - PubMed
McNicholas 2012
Okusanya 2013
-
- Okusanya BO, Aigere EOS, Abe A, Ibrahim HM, Salawu RA. Maternal deaths: Initial report of an on‐going monitoring of maternal deaths at the Federal Medical Centre Katsina, Northwest Nigeria. Journal of Maternal‐Fetal and Neonatal Medicine 2013; Vol. 26, issue 9:885‐8. - PubMed
PIP 1995
-
- Population Information Program. IUDs ‐ an update. Population Reports 1995;Series B(6):1‐35. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Saav 2012
Sinei 1990
-
- Sinei SK, Schulz KF, Lamptey PR, Grimes DA, Mati JK, Rosenthal SM, et al. Preventing IUCD‐related pelvic infection: the efficacy of prophylactic doxycycline at insertion. British Journal of Obstetrics and Gynaecology 1990;97(5):412‐9. - PubMed
Sivin 1981
-
- Sivin I, Tatum HJ. Four years of experience with the TCu 380A intrauterine contraceptive device. Fertility and Sterility 1981;36(2):159‐63. - PubMed
Souza 2013
-
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381(9879):1747‐55. - PubMed
Stanback 1997
-
- Stanback J, Thompson A, Hardee K, Janowitz B. Menstruation requirements: a significant barrier to contraceptive access in developing countries. Studies in Family Planning 1997;28(3):245‐50. - PubMed
Stanek 2009
-
- Stanek AM, Bednarek PH, Nichols MD, Jensen JT, Edelman AB. Barriers associated with the failure to return for intrauterine device insertion following first‐trimester abortion. Contraception 2009;79(3):216‐20. - PubMed
Uzelac 2007
-
- Uzelac PS, Garmel SH. Early pregnancy risks. In: Decherney AH, Nathan L, Goodwin TM, Laufer N editor(s). Current Diagnosis & Treatment. Obstetrics and Gynecology. 10th Edition. New York, USA: The McGraw‐Hill Companies, 2007:259‐272.
Walsh 1998
-
- Walsh T, Grimes D, Frezieres R, Nelson A, Bernstein L, Coulson A, et al. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet 1998;351(9108):1005‐8. - PubMed
WHO 1987
-
- World Health Organization. Mechanism of action, safety and efficacy of intrauterine devices. Technical Report Series 753. Geneva: World Health Organization, 1987. - PubMed
WHO 1994
-
- World Health Organization. A randomized multicentre trial of the Multiload 375 and TCu380A IUDs in parous women: three‐year results. Contraception 1994;49(6):543‐9. - PubMed
Wolf 1994
-
- Wolf M, Benson J. Meeting women's needs for post‐abortion family planning. Report of a Bellagio Technical Working Group. International Journal of Gynaecology and Obstetrics 1994;45(Suppl):S1‐S23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

